The Adsorption of Dextranase onto Mg/Fe-Layered Double Hydroxide: Insight into the Immobilization
Abstract
:1. Introduction
2. Results
2.1. Adsorption Study
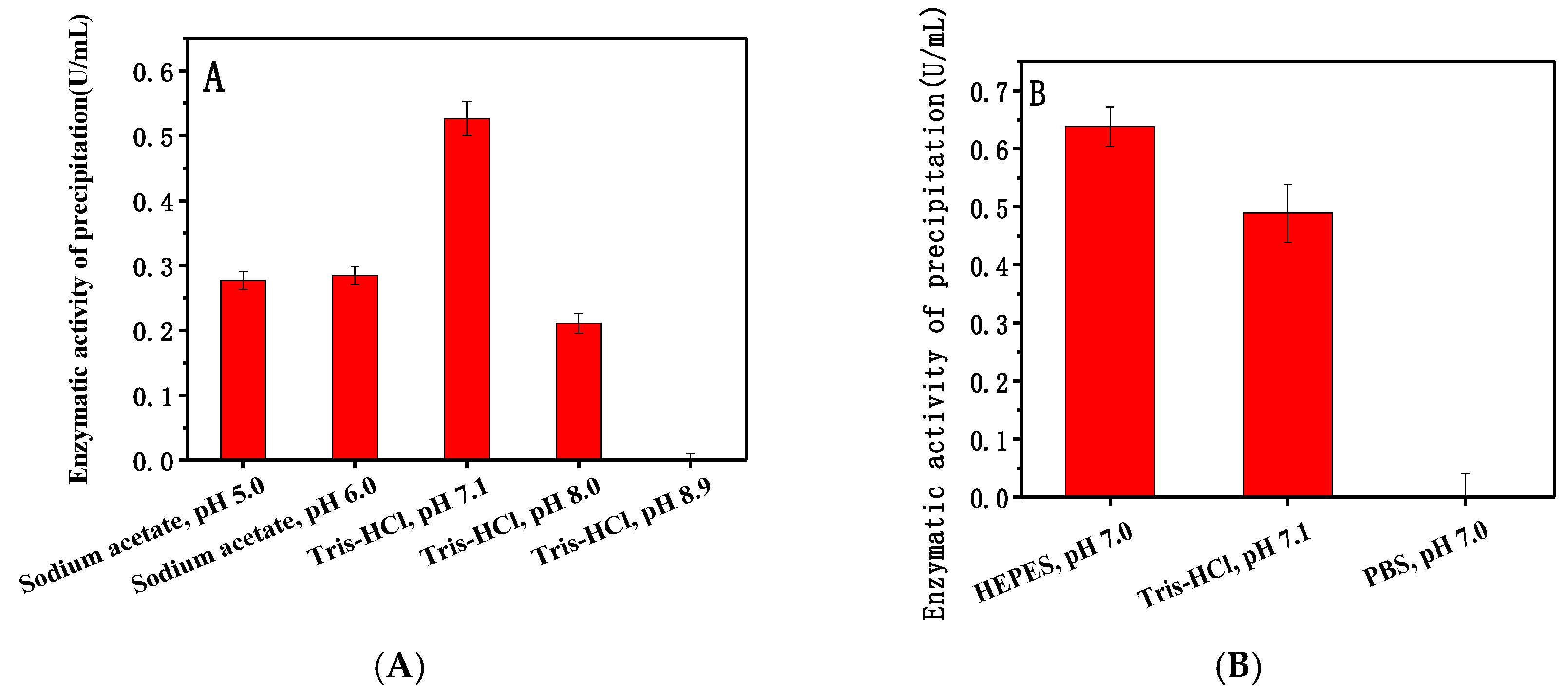
2.1.1. Optimum Buffers for Adsorption
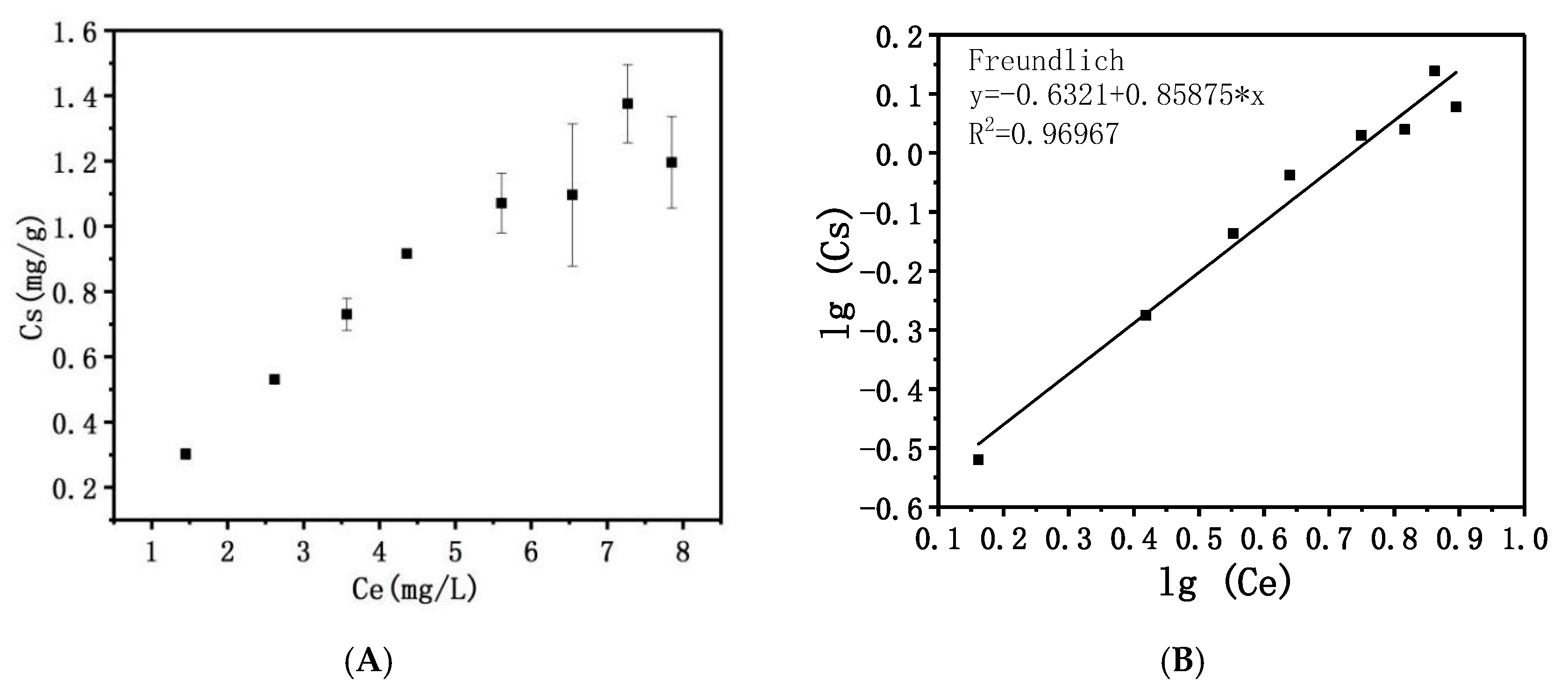
2.1.2. Adsorption Isotherm
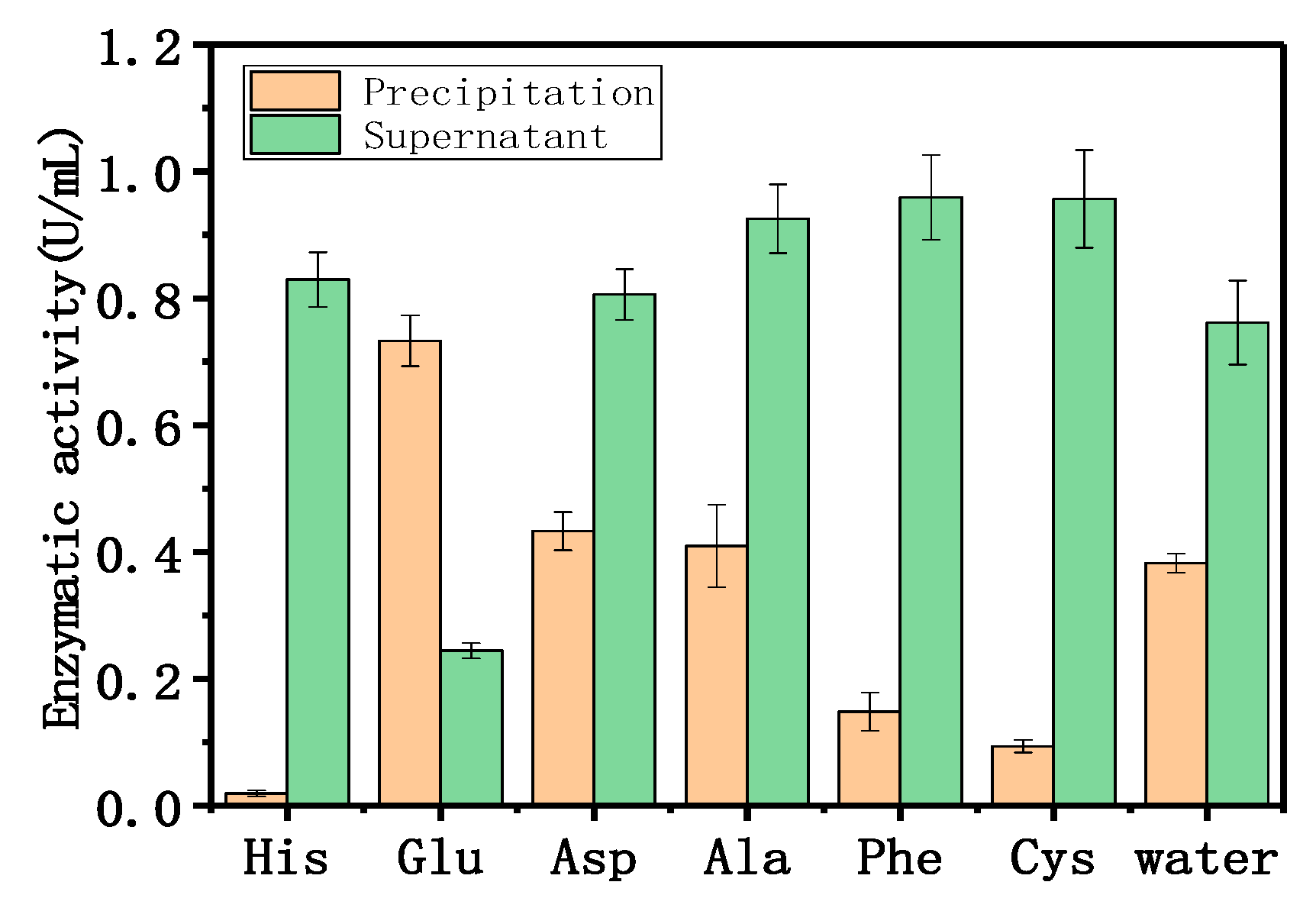
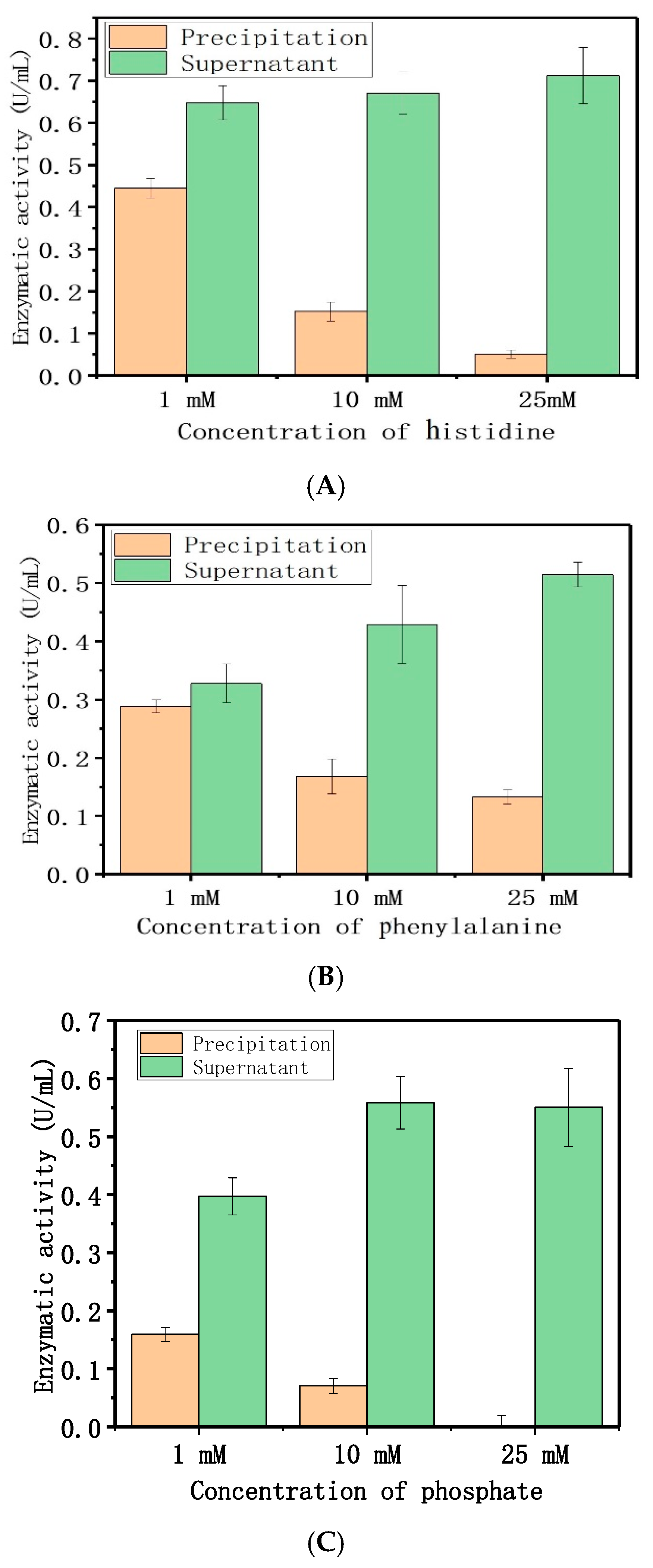
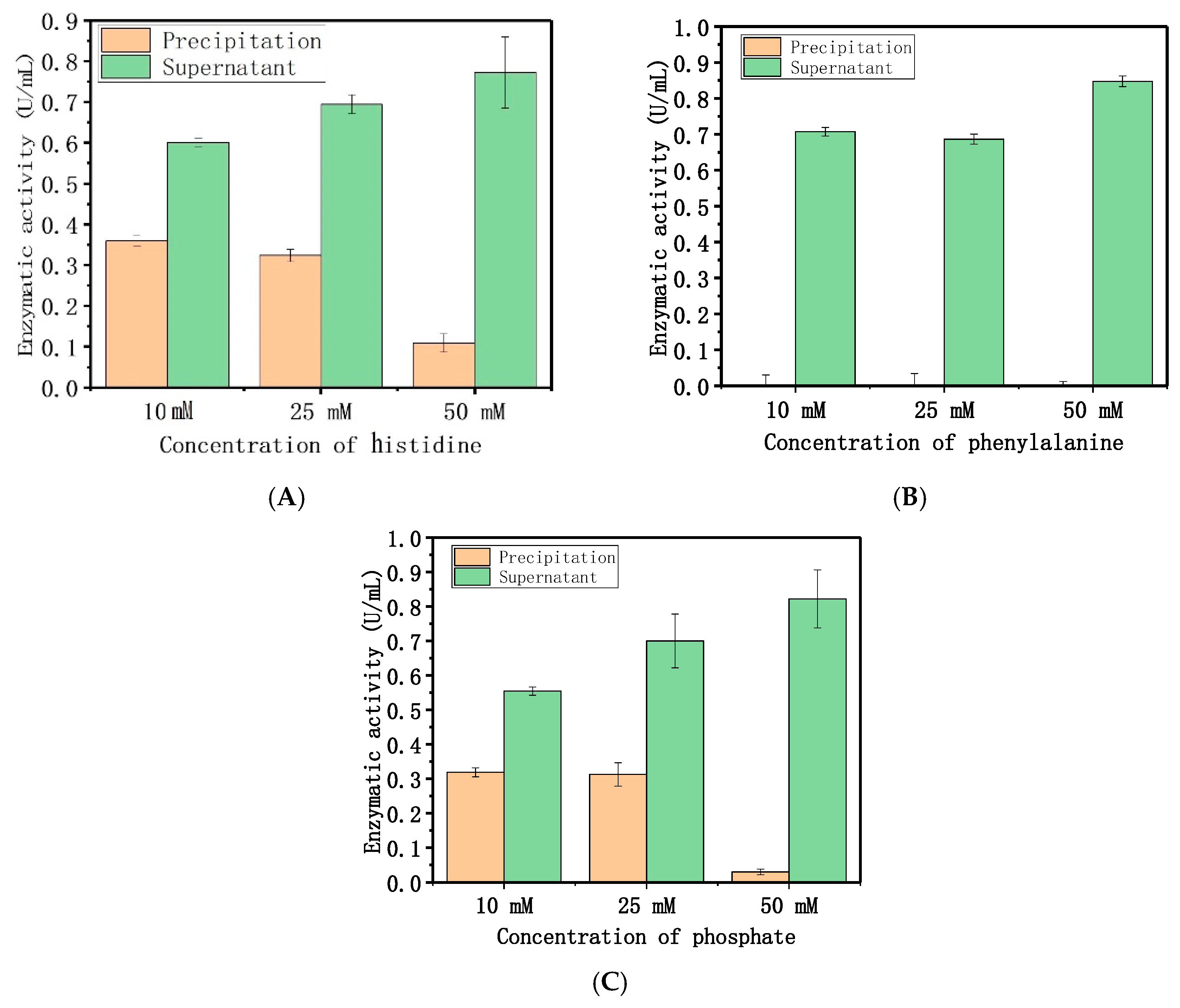
2.1.3. Adsorption Mechanism
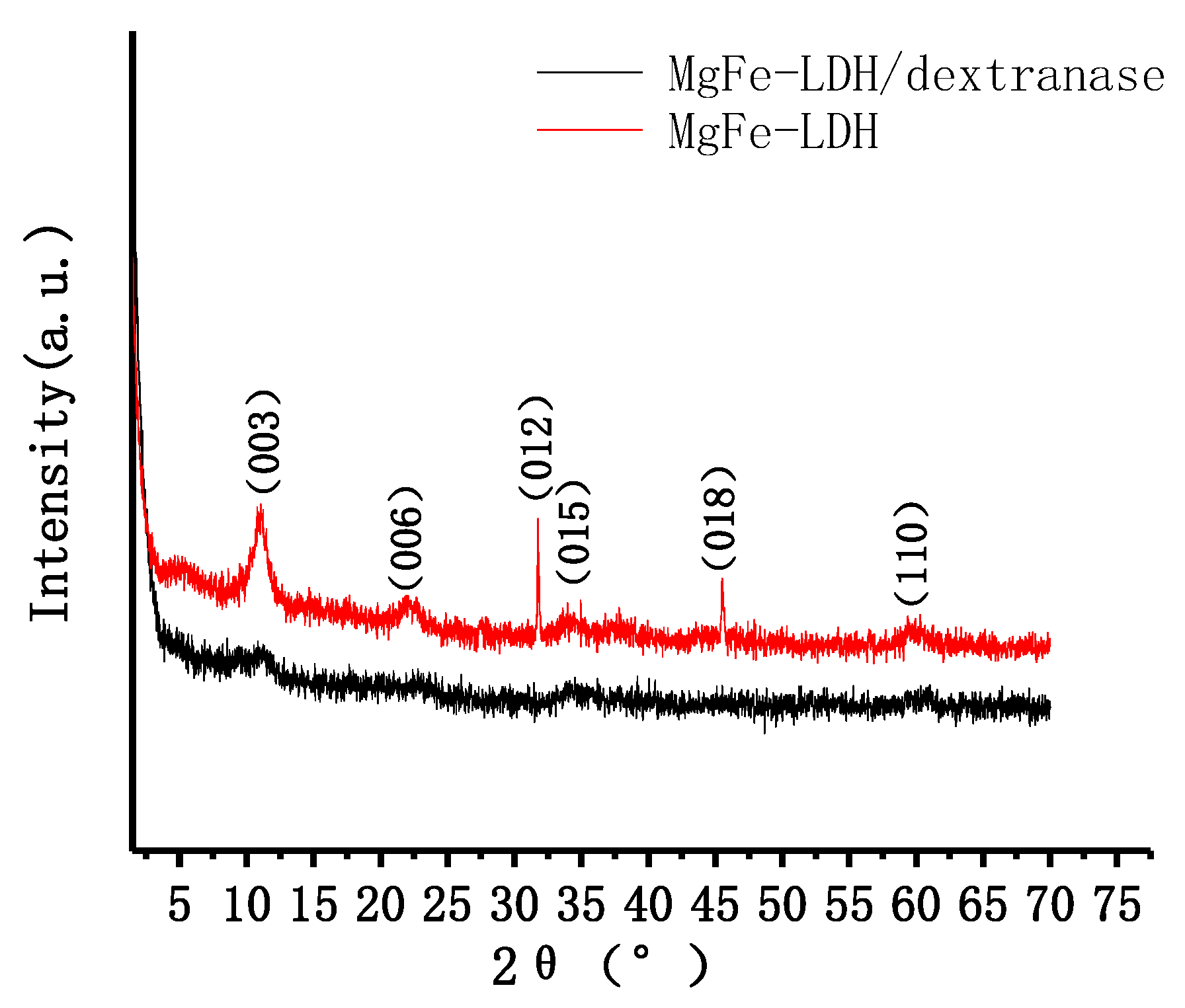
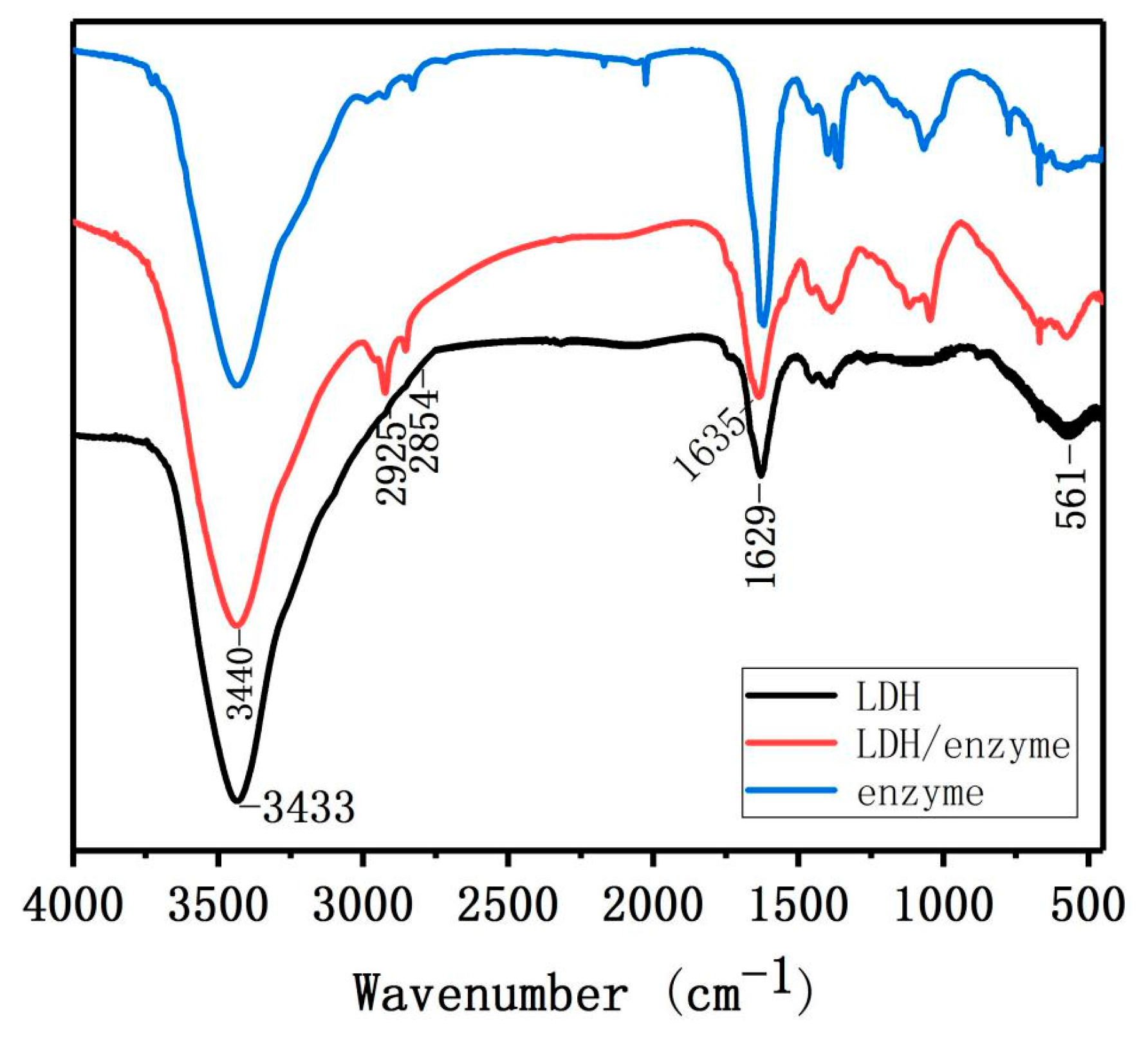
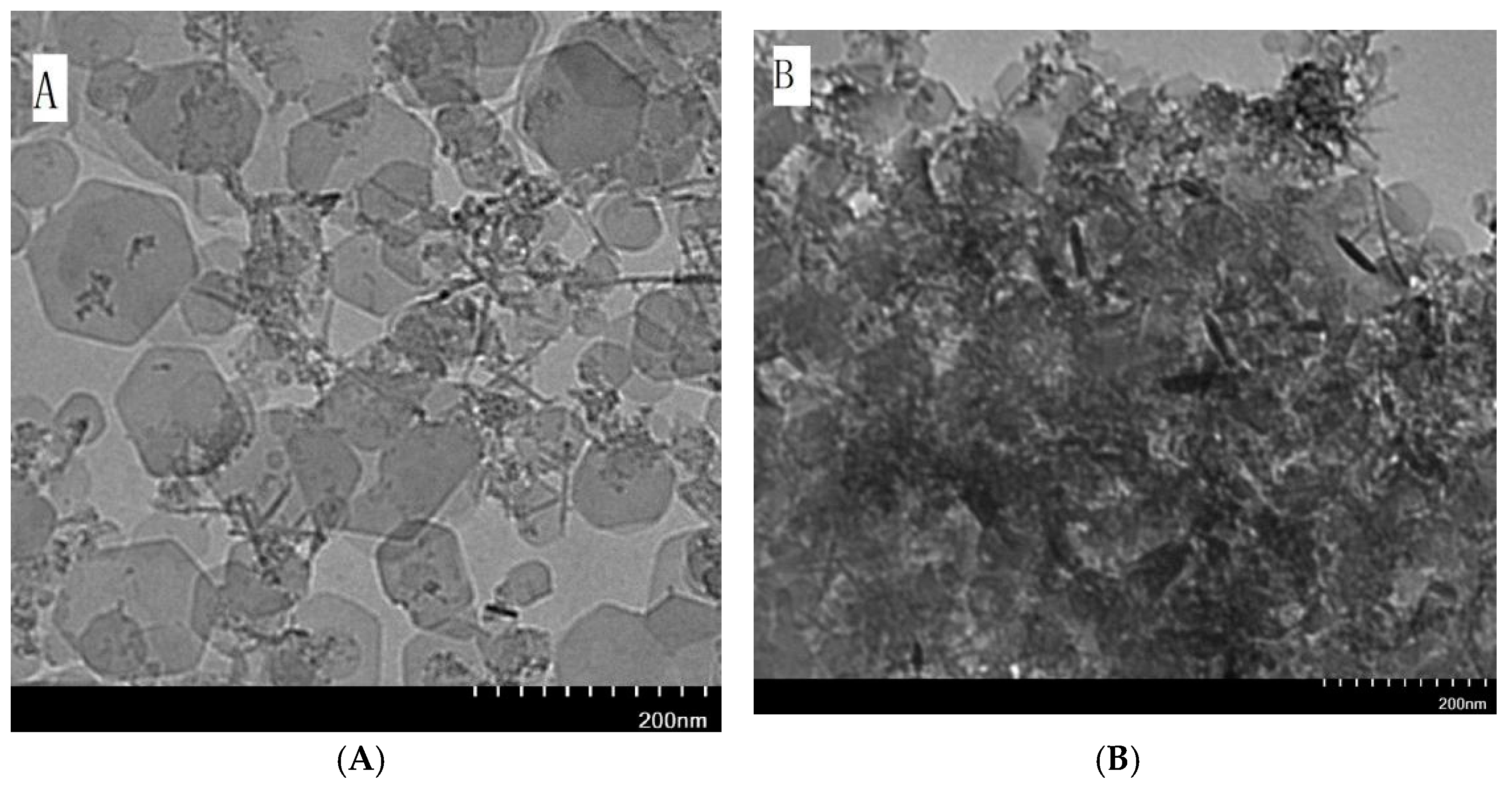
2.2. Characterization
3. Discussion
4. Materials and Methods
4.1. Starting Materials
4.2. Preparation of Dextranase
4.3. Dextranase Activity Assay
4.4. Synthesis of Mg/Fe-LDH
4.5. Adsorption Studies
4.5.1. The Synthesis of a Mg/Fe-LDH/Dextranase Biohybrid via Co-Precipitation
4.5.2. Determination and Quantitation of Adsorption
4.5.3. Optimum Buffer for Adsorption
4.5.4. Adsorption Isotherm
4.5.5. Adsorption Mechanism
4.6. Chemical Physical Characteristics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Human and Animal Rights
References
- Jin, W.; Brennan, J.D. Properties and Applications of Proteins Encapsulated Within Sol–Gel-Derived Materials. ChemInform 2010, 461, 1–36. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Park, J.S.; Jeong, Y.J. Cellular uptake behavior of [γ-32P] labeled ATP–LDH nanohybrids. J. Mater. Chem. 2001, 11, 1671–1674. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Park, J.S.; Jeong, Y.J.; Portier, J. Intercalative Nanohybrids of Nucleoside Monophosphates and DNA in Layered Metal Hydroxide. J. Am. Chem. Soc. 1999, 121, 1399–1400. [Google Scholar] [CrossRef]
- Rives, V. Layered double hydroxides: Present and future. Cheminform 2001, 37, 193–223. [Google Scholar]
- Khan, A.I.; O’Hare, D. Intercalation Chemistry of Layered Double Hydroxides: Recent Developments and Applications. Cheminform 2003, 34, 3191–3198. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar]
- Hajibeygi, M.; Shabanian, M.; Omidi-Ghallemohamadi, M. Development of new acid-imide modified Mg-Al/LDH reinforced semi-crystalline poly(amide-imide) containing naphthalene ring; study on thermal stability and optical properties. Appl. Clay Sci. 2017, 139, 9–19. [Google Scholar] [CrossRef]
- Olfs, H.W.; Torres-Dorante, L.O.; Eckelt, R.; Kosslick, H. Comparison of different synthesis routes for Mg-Al layered double hydroxides (LDH): Characterization of the structural phases and anion exchange properties. Appl. Clay Sci. 2009, 43, 459–464. [Google Scholar] [CrossRef]
- Shan, D.; Cosnier, S.; Mousty, C. HRP/[Zn–Cr–ABTS] redox clay-based biosensor: Design and optimization for cyanide detection. Biosens. Bioelectron. 2004, 20, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Prevot, V.; Tokudome, Y. 3D hierarchical and porous layered double hydroxide structures: An overview of synthesis methods and applications. J. Mater. Sci. 2017, 52, 11229–11250. [Google Scholar] [CrossRef]
- Djebbi, M.A.; Braiek, M.; Hidouri, S.; Namour, P.; Jaffrezic-Renault, N.; Amara, A.B. Novel biohybrids of layered double hydroxide and lactate dehydrogenase enzyme: Synthesis, characterization and catalytic activity studies. J. Mol. Struct. 2016, 1105, 381–388. [Google Scholar] [CrossRef]
- Djebbi, M.A.; Charradi, K.; Amara, A.B.; Rhaiem, H.B. Immobilization of LDh enzyme on Layered Double Hydroxides: Structural and morphological modification. In Proceedings of the 2014 International Conference on Composite Materials & Renewable Energy Applications (ICCMREA), Sousse, Tunisia, 22–24 January 2014; pp. 1–7. [Google Scholar]
- Conterosito, E.; Van Beek, W.; Palin, L.; Croce, G.; Perioli, L.; Viterbo, D.; Gatti, G.; Milanesio, M. Development of a Fast and Clean Intercalation Method for Organic Molecules into Layered Double Hydroxides. Cryst. Growth Des. 2013, 13, 1162–1169. [Google Scholar] [CrossRef]
- Toson, V.; Milanesio, M.; Conterosito, E. Crystal packing and layered morphology relationships in naphthalene sulfonate compounds. Z. Krist. Crystall. Mater. 2017, 232, 463–469. [Google Scholar] [CrossRef]
- Zohra, R.R.; Aman, A.; Zohra, R.R.; Ansari, A.; Ghani, M.; Qader, S.A. Dextranase: Hyper production of dextran degrading enzyme from newly isolated strain of Bacillus licheniformis. Carbohydr. Polym. 2013, 92, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G.; Monge, A. Optimization of sugarcane factory application of commercial dextranases. Process Biochem. 2005, 40, 1881–1894. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ko, E.A.; Kang, H.K.; Kim, D. Construction, expression and characterization of fusion enzyme from Arthrobacter oxydans dextranase and Klebsiella pneumoniae amylase. Biotechnol. Lett. 2009, 31, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Bashari, M.; Eibaid, A.; Wang, J.; Tian, Y.; Xu, X.; Jin, Z. Influence of low ultrasound intensity on the degradation of dextran catalyzed by dextranase. Ultrason. Sonochem. 2013, 20, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Millson, S.H.; Evans, I.H. Multiple dextranases from the yeast Lipomyces starkeyi. Antonie Van Leeuwenhoek 2007, 92, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.R.; Ferretti, J.J. Nucleotide sequence of the dextran glucosidase (dexB) gene of Streptococcus mutans. J. Gener. Microbiol. 1990, 136, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial Dextran-Hydrolyzing Enzymes: Fundamentals and Applications. Microbiol. Mol. Biol. Rev. 2005, 69, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, M.; Wang, S.; Jiao, Y.; Li, W.; Zhu, Q.; Liu, Z. Purification and characterization of a novel marine Arthrobacter oxydans KQ11 dextranase. Carbohydr. Polym. 2014, 106, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, M.; Wang, X.; Jiao, Y.; Fang, Y.; Liu, Z.; Wang, S. Improving stability of a novel dextran-degrading enzyme from marine Arthrobacter oxydans KQ11. Carbohydr. Polym. 2014, 103, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Aslan, Y.; Tanriseven, A. Immobilization of Penicillium lilacinum dextranase to produce isomaltooligosaccharides from dextran. Biochem. Eng. J. 2007, 34, 8–12. [Google Scholar] [CrossRef]
- Erhardt, F.A.; Jördening, H.J. Immobilization of dextranase from Chaetomium erraticum. J. Biotechnol. 2007, 131, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 391–396. [Google Scholar] [CrossRef]
- Gondim, D.R.; Cecilia, J.A.; Santos, S.O.; Rodrigues, T.N.; Aguiar, J.E.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Azevedo, D.C.; Silva, I.J., Jr. Influence of buffer solutions in the adsorption of human serum proteins onto layered double hydroxide. Int. J. Biol. Macromol. 2018, 106, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.W.; Botstein, D.; Roth, J.R. Advanced Bacterial Genetics: A Manual for Genetic Engineering; Koros Press: Cold Spring Harbor, NY, USA, 1980. [Google Scholar]
- Crowe, J.; Masone, B.S.; Ribbe, J. One-step purification of recombinant proteins with the 6xHis tag and Ni-NTA resin. Mol. Biotechnol. 1995, 4, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In situ investigation of the thermal decomposition of Co–Al hydrotalcite in different atmospheres. J. Mater. Chem. 2001, 11, 821–830. [Google Scholar] [CrossRef]
- Sass, C.; Briand, M.; Benslimane, S.; Renaud, M.; Briand, Y. Characterization of rabbit lactate dehydrogenase-M and lactate dehydrogenase-H cDNAs. Control of lactate dehydrogenase expression in rabbit muscle. J. Biol. Chem. 1989, 264, 4076–4081. [Google Scholar] [PubMed]
- Pereira, A.C.; Aguiar, M.R.; Kisner, A.; Macedo, D.V.; Kubota, L.T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B Chem. 2007, 124, 269–276. [Google Scholar] [CrossRef]
- Chaiet, L.; Kempf, A.J.; Harman, R.; Kaczka, E.; Weston, R.; Nollstadt, K.; Wolf, F.J. Isolation of a pure dextranase from Penicillium funiculosum. Appl. Microbiol. 1970, 20, 421–426. [Google Scholar] [PubMed]
- Wynter, C.V.; Chang, M.; De Jersey, J.; Patel, B.; Inkerman, P.A.; Hamilton, S. Isolation and characterization of a thermostable dextranase. Enzym. Microb. Technol. 1997, 20, 242–247. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on Sugar Determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar]
- Lever, M. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 1972, 47, 273–279. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Lipin, V.A.; Danilov, V.I.; Kuznetzov, A.A. Special Requirements to Aluminium Hydroxide of Non-Metallurgical Application; Anon, Light Metals; TMS: Pittsburgh, PA, USA, 2002; pp. 169–173. [Google Scholar]
- Arai, Y.; Ogawa, M. Preparation of Co–Al layered double hydroxides by the hydrothermal urea method for controlled particle size. Appl. Clay Sci. 2009, 42, 601–604. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Liu, L.; Fang, Y.; Zhang, X.; Lyu, M.; Wang, S. The Adsorption of Dextranase onto Mg/Fe-Layered Double Hydroxide: Insight into the Immobilization. Nanomaterials 2018, 8, 173. https://doi.org/10.3390/nano8030173
Ding Y, Liu L, Fang Y, Zhang X, Lyu M, Wang S. The Adsorption of Dextranase onto Mg/Fe-Layered Double Hydroxide: Insight into the Immobilization. Nanomaterials. 2018; 8(3):173. https://doi.org/10.3390/nano8030173
Chicago/Turabian StyleDing, Yi, Le Liu, Yaowei Fang, Xu Zhang, Mingsheng Lyu, and Shujun Wang. 2018. "The Adsorption of Dextranase onto Mg/Fe-Layered Double Hydroxide: Insight into the Immobilization" Nanomaterials 8, no. 3: 173. https://doi.org/10.3390/nano8030173



