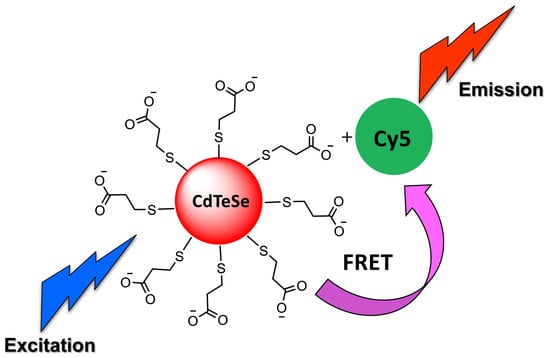
NIR-Emitting Alloyed CdTeSe QDs and Organic Dye Assemblies: A Nontoxic, Stable, and Efficient FRET System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Synthesis of MPA-Capped CdTeSe QDs
2.3. MPA-Capped CdTeSe QDs and Dye Conjugates
2.4. Titration of MPA-CdTeSe QDs with Cy5 Dye
2.5. CCK-8 Assay
2.6. Confocal Microscopy
3. Results and Discussion
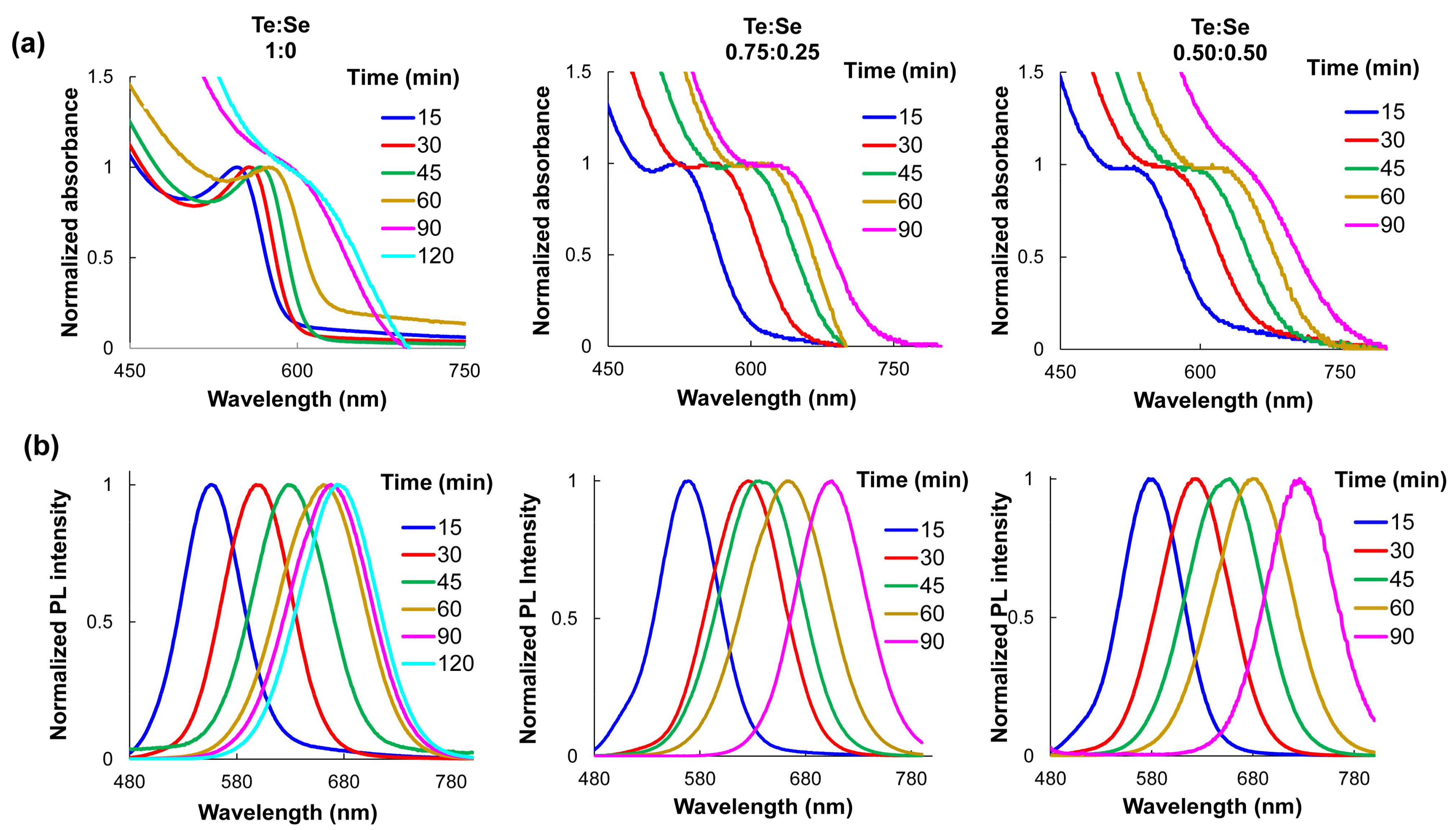
3.1. Synthesis of Near-Infrared MPA-Capped CdTe and CdTeSe QDs
Characterization by UV-VIS and Fluorescence Spectroscopy
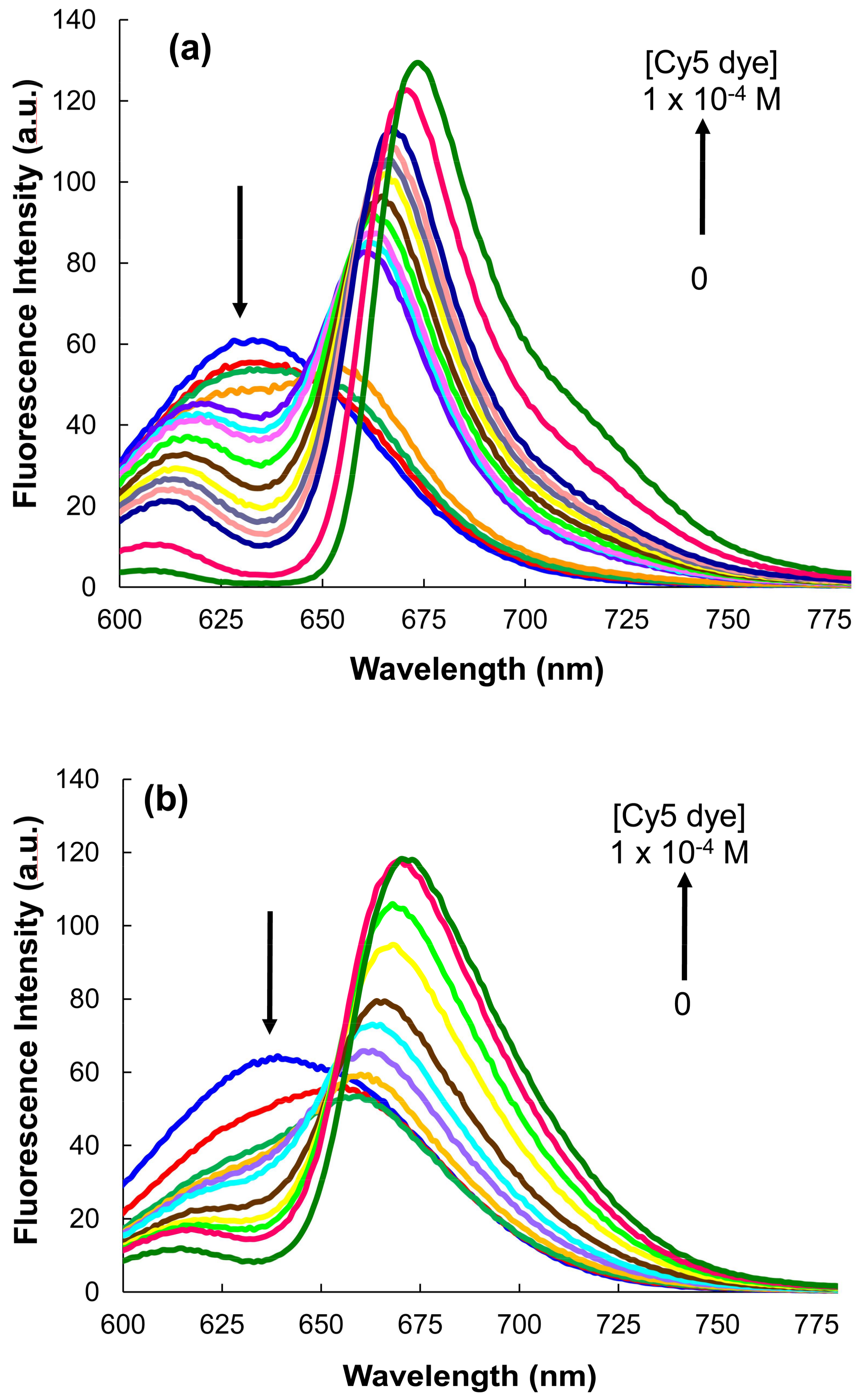
3.2. Förster Resonance Energy Transfer FRET Studies
3.3. Selection of the Optimal Conditions for the FRET Process
3.4. Zeta Potential
3.5. MPA-Capped CdTeSe QDs’ Titrations of Different Sizes with Cy5 Dye
3.6. Citocompatibility of the CdTeSe/MPA QDs
3.7. Cell Internalization of the MPA-Capped CdTeSe QDs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, A.M.; Nie, S.M. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Rao, J. Quantum Dot Bioconjugates for in vitro Diagnostics & in vivo Imaging. Cancer Biomark. 2008, 4, 307–319. [Google Scholar] [PubMed]
- Medintz, I.L.; Mattoussi, H.; Clapp, A.R. Potential Clinical Applications of Quantum Dots. Int. J. Nanomed. 2008, 3, 151–167. [Google Scholar]
- Michalet, X.; Pinaud, F.; Bentolila, L.; Tsay, J.; Doose, S.; Li, J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Robel, I.; Subramanian, V.; Kuno, M.; Kamat, P.V. Quantum Dot Solar Cells. Harvesting Light Energy with CdSe Nanocrystals Molecularly Linked to Mesoscopic TiO2 Films. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.A.; Konstantatos, G.; Zhang, S.; Cyr, P.W.; Klem, E.J.D.; Levina, L.; Sargent, E.H. Solution-processed PbS Quantum Dot Infrared Photodetectors and Photovoltaics. Nat. Mater. 2005, 4, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In vivo near-infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, P.; Zhang, L. Synthesis of Near-Infrared-Emitting CdTeSe and CdZnTeSe Quantum Dots. Luminescence 2012, 28, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Cassette, E.; Helle, M.; Bezdetnaya, L.; Marchal, F.; Dubertret, B.; Pons, T. Design of New Quantum Dot Materials for Deep Tissue Infrared Imaging. Adv. Drug Deliv. Rev. 2013, 65, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.E.; Nie, S. Alloyed Semiconductor Quantum Dots: Tuning the Optical Properties without Changing the Particle Size. J. Am. Chem. Soc. 2003, 125, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.E.; Strausburg, J.B.; Nie, S. A New Class of Far-Red and Near-Infrared Biological Labels Based on Alloyed Semiconductor Quantum Dots. J. Nanosci. Nanotechnol. 2004, 4, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Singhal, A.; Wang, C.; Chan, W.C.W. Optimizing the Synthesis of Red- to Near-IR-Emitting CdS-Capped CdTexSe1−x Alloyed Quantum Dots for Biomedical Imaging. Chem. Mater. 2006, 18, 4845–4854. [Google Scholar] [CrossRef]
- Pons, T.; Lequeux, N.; Mahler, B.; Sasnouski, S.; Fragola, A.; Dubertret, B. Synthesis of Near-Infrared-Emitting, Water-Soluble CdTeSe/CdZnS Core/Shell Quantum Dots. Chem. Mater. 2009, 21, 1418–1424. [Google Scholar] [CrossRef]
- Wang, R.; Calvignanello, O.; Ratcliffe, C.I.; Wu, X.; Leek, D.M.; Zaman, M.B.; Kingston, D.; Ripmeester, J.A.; Yu, K. Homogeneously-Alloyed CdTeSe Single-Sized Nanocrystals with Bandgap Photoluminescence. J. Phys. Chem. C 2009, 113, 3402–3408. [Google Scholar] [CrossRef]
- Talapin, D.V.; Mekis, I.; Gotzinger, S.; Kornowski, A.; Benson, O.; Weller, H. CdSe/CdS/ZnS and CdSe/ZnSe/ZnS Core-Shell-Shell Nanocrystals. J. Phys. Chem. B 2004, 108, 18826–18831. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface Modification, Functionalization and Bioconjugation of Colloidal Inorganic Nanoparticles. Philos. Trans. R. Soc. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.G.; Bai, J.Y.; Fang, T.; Guo, H.Q. Synthesis of High-Quality Water-Soluble Near-Infrared-Emitting CdTe Quantum Dots Capped with 3-Mercaptobutyric Acid. J. Nanosci. Nanotechnol. 2014, 14, 4940–4948. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.G.; Fang, T.; Bai, J.Y.; Guo, H.Q. Regulating Properties of Quantum Dots: Effect of Methyl Side Groups of Mercapto Acids. RSC Adv. 2013, 3, 4935–4939. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Qiao, Y.; Su, X. One-Pot Synthesis of Ternary CuInS2 Quantum Dots with Near-Infrared Fluorescence in Aqueous Solution. RSC Adv. 2012, 2, 819–825. [Google Scholar] [CrossRef]
- Su, Y.; He, Y.; Lu, H.; Sai, L.; Li, Q.; Li, W.; Wang, L.; Shen, P.; Huang, Q.; Fan, C. The Cytotoxicity of Cadmium Based, Aqueous Phase-Synthesized, Quantum Dots and its Modulation by Surface Coating. Biomaterials 2009, 30, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhao, K.; Wang, J.; Zhang, H.; Feng, Y.; Zhong, X. Near Infrared Absorption of CdSexTe1−x Alloyed Quantum Dot Sensitized Solar Cells with More than 6% Efficiency and High Stability. ACS Nano 2013, 7, 5215–5222. [Google Scholar] [CrossRef] [PubMed]
- Peynshaert, K.; Soenen, S.J.; Manshian, B.B.; Doak, S.H.; Braeckmans, K.; de Smedt, S.C.; Remaut, K. Coating of Quantum Dots Strongly Defines their Effect on Lysosomal Health and Autophagy. Acta Biomater. 2017, 48, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hub, M.; Fan, C.; He, Y.; Li, Q.; Li, W.; Wang, L.-H.; Shen, P.; Huang, Q. The Cytotoxicity of CdTe Quantum Dots and the Relative Contributions from Released Cadmium Ions and Nanoparticle Properties. Biomaterials 2010, 31, 4829–4834. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, R.; Liu, J.; Zhang, B.; Wang, Y.; Liu, X.; Law, W.-C.; Liu, L.; Ye, L.; Yong, K.-T. Cytotoxicity Assessment of Functionalized CdSe, CdTe and InP Quantum Dots in Two Human Cancer Cell Models. Mater. Sci. Eng. C 2015, 57, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, Y.; Su, Y.; Li, X.; Huang, Q.; Wang, H.; Zhang, X.; Tai, R.; Fan, C. The Cytotoxicity of Cadmium-Based Quantum Dots. Biomaterials 2012, 33, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, X.; Wang, S.; Wu, Y.; Hao, X.; Xu, C.; Zhao, X. Microwave-Assisted Synthesis of Highly Luminescent Glutathione-Capped Zn1−xCdxTe Alloyed Quantum Dots with Excellent Biocompatibility. J. Mater. Chem. 2012, 22, 11390–11395. [Google Scholar] [CrossRef]
- Xue, B.; Deng, D.-W.; Cao, J.; Liu, F.; Li, X.; Akers, W.; Achilefu, S.; Gu, Y.-Q. Synthesis of NAC Capped Near Infrared-Emitting CdTeS Alloyed Quantum Dots and Application for in vivo Early Tumor Imaging. Dalton Trans. 2012, 41, 4935–4947. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Clapp, A.R.; Mattoussi, H.; Goldman, E.R.; Fisher, B.; Mauro, J.M. Self-Assembled Nanoscale Biosensors Based on Quantum Dot FRET Donors. Nat. Mater. 2003, 2, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for Fluorescence Resonance Energy Transfer Analysis: Beyond Traditional Donor-Acceptor Combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4588. [Google Scholar] [CrossRef] [PubMed]
- Oluwatobi, O.; Daramola, O.A.; Ncapayi, V. A Facile Green Synthesis of Type II Water Soluble CdTe/CdS Core Shell Nanoparticles. Mater. Lett. 2014, 133, 9–13. [Google Scholar]
- Yu, W.W.; Qu, L.H.; Guo, W.Z.; Peng, X.G. Experimental determination of the extinction coefficient of CdTe, CdSe and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Rodríguez-Velázquez, E.; Silva, M.; Taboada, P.; Mano, J.F.; Suárez-Quintanilla, D.; Alatorre-Meda, M. Enhanced Cell Affinity of Chitosan Membranes Mediated by Superficial Cross-Linking: A Straightforward Method Attainable by Standard Laboratory Procedures. Biomacromolecules 2014, 15, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-Assembly of CdSe-ZnS Quantum Dot Bioconjugates Using an Engineered Recombinant Protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Pathak, S.; Choi, S.K.; Arnheim, N.; Thompson, M.E. Hydroxylated Quantum Dots as Luminescent Probes for in Situ Hybridization. J. Am. Chem. Soc. 2001, 123, 4103–4104. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Huang, S.; Su, W.; Chan, W.H.; Liu, Y. Facile Synthesis and Characterization of Highly Fluorescent and Biocompatible N-acetyl-l-cysteine Capped CdTe/CdS/ZnS core/shell/shell Quantum Dots in Aqueous Phase. Nanotechnology 2012, 23, 495717. [Google Scholar] [CrossRef] [PubMed]
- Borchert, H.; Talapin, D.V.; Gaponik, N.; McGinley, C.; Adam, S.; Lobo, A.; Moller, T.; Weller, H. Relations between the Photoluminescence Efficiency of CdTe Nanocrystals and Their Surface Properties Revealed by Synchrotron XPS. J. Phys. Chem. B 2003, 107, 9662–9668. [Google Scholar] [CrossRef]
- Oda, M.; Tsukamoto, J.; Hasegawa, A.; Iwami, N.; Nishiura, K.; Hagiwara, I.; Ando, N.; Horiuchi, H.; Tani, T. Photobrightening of CdSe/ZnS/TOPO Nanocrystals. J. Lumin. 2007, 122–123, 762–765. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Mauro, J.M.; Fisher, B.R.; Bawendi, M.G.; Mattoussi, H. Fluorescence Resonance Energy Transfer between Quantum Dot Donors and Dye-Labeled Protein Acceptors. J. Am. Chem. Soc. 2004, 126, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.Z.; Matthews, D.R.; Summers, H.D.; Njoh, K.L.; Errington, R.J.; Smith, P.J. Development of FRET-Based Assays in the Far-Red Using CdTe Quantum Dots. J. Biomed. Biotechnol. 2007, 2007, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; p. 678. [Google Scholar]
- Peng, L.; He, M.; Chen, B.; Wu, Q.; Zhang, Z.; Pang, D.; Zhu, Y.; Hu, B. Cellular Uptake, Elimination and Toxicity of CdSe/ZnS Quantum Dots in HepG2 Cells. Biomaterials 2013, 34, 9545–9558. [Google Scholar] [CrossRef] [PubMed]
- Kauffer, F.A.; Merlin, C.; Balan, L.; Schneider, R. Incidence of the Core Composition on the Stability, the ROS Production and the Toxicity of CdSe Quantum Dots. J. Hazard. Mater. 2014, 268, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, C.C.; Park, S.J.; Chapman, J.D. The Radiation Hypersensitivity of Cells at Mitosis. Int. J. Rad. Biol. 2002, 78, 1149–1157. [Google Scholar] [CrossRef] [PubMed]







| MPA-QDs | λem (nm) | DUV (nm) | J (cm3Lmol−1) | E | R0 (nm) | r (nm) | K |
|---|---|---|---|---|---|---|---|
| QD629 | 629 | 3.48 | 2.01 × 10−12 | 0.96 | 4.37 | 2.58 | 2.30 × 104 |
| QD636 | 636 | 3.63 | 1.88 × 10−12 | 0.88 | 4.41 | 3.14 | 8.24 × 104 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Herrera, D.E.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Paraguay-Delgado, F.; Tirado-Guízar, A.; Taboada, P.; Pina-Luis, G. NIR-Emitting Alloyed CdTeSe QDs and Organic Dye Assemblies: A Nontoxic, Stable, and Efficient FRET System. Nanomaterials 2018, 8, 231. https://doi.org/10.3390/nano8040231
Ramírez-Herrera DE, Rodríguez-Velázquez E, Alatorre-Meda M, Paraguay-Delgado F, Tirado-Guízar A, Taboada P, Pina-Luis G. NIR-Emitting Alloyed CdTeSe QDs and Organic Dye Assemblies: A Nontoxic, Stable, and Efficient FRET System. Nanomaterials. 2018; 8(4):231. https://doi.org/10.3390/nano8040231
Chicago/Turabian StyleRamírez-Herrera, Doris E., Eustolia Rodríguez-Velázquez, Manuel Alatorre-Meda, Francisco Paraguay-Delgado, Antonio Tirado-Guízar, Pablo Taboada, and Georgina Pina-Luis. 2018. "NIR-Emitting Alloyed CdTeSe QDs and Organic Dye Assemblies: A Nontoxic, Stable, and Efficient FRET System" Nanomaterials 8, no. 4: 231. https://doi.org/10.3390/nano8040231