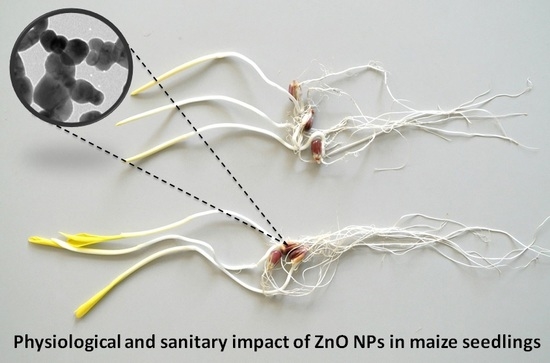
Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis Protocol
2.3. ZnO NP Characterization
2.3.1. UV–Vis
2.3.2. Optical Absorption Properties
2.3.3. Fluorescence
2.3.4. Transmission Electron Microscopy
2.3.5. Nanoparticle Tracking Analysis
2.3.6. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflection
2.4. Laboratory Experiments
2.4.1. Maize Seed
2.4.2. Seed Conditioning with ZnO NPs
2.4.3. Standard Germination and Accelerated Aging Tests
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. ZnO NP Characterization
3.1.1. Optical Properties
3.1.2. Morphology of ZnO NPs
3.1.3. Particle Size and Particle Concentration Using NTA
3.1.4. FTIR-ATR Analysis
3.2. Maize Seed Coating Studies
3.2.1. Physiological and Sanitary Quality of Maize Seeds
3.2.2. Maize Seedling Vigor Evaluation
3.2.3. FTIR-ATR Studies (Shoots and Roots)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tannahill, R. Food in History, Revised ed.; Crown Publishers: New York, NY, USA, 1988; p. 424. ISBN 9780517884041. [Google Scholar]
- Goodman, M.; Stuber, C. Isozymatic and morphological diversity in the races of maize of Mexico. Econ. Bot. 2000, 54, 43–59. [Google Scholar]
- Dyer, G.A.; Taylor, J.E. A crop population perspective on maize seed systems in Mexico. Proc. Natl. Acad. Sci. USA 2008, 105, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.L.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Available online: https://www.gob.mx/siap (accessed on 27 February 2018).
- Paulino-Flores, M.; Martínez-Campos, Á.R.; Martínez-Castañeda, F.E.; López-Orona, C.A.; Vizcarra-Bordi, I.; Munguía, N. Evaluation of the sustainability of hybrid and native maize production systems. J. Clean. Prod. 2017, 150, 287–293. [Google Scholar] [CrossRef]
- Cruz-Delgado, S.; Gómez-Valdez, M.; Ortiz-Pulido, M.; Entzana-Tadeo, A.M.; Suárez-Hernández, C.Y.; Santillán-Moctezuma, V. Situación Actual y Perspectivas del Maíz en México 1996–2012; SAGARPA: Santa Cruz Atoyac, Mexico, 2008; p. 130.
- Cromwell, E. Seed Diffusion Mechanisms in Small Farmer Communities. Lessons from Asia, Africa and Latin America, Agricultural Administration Unit ed.; Overseas Development Institute: London, UK, 1990; p. 57. ISSN 0951-1873. [Google Scholar]
- Auld, D.S. Zinc coordination sphere in biochemical zinc sites. In Zinc Biochemistry, Physiology, and Homeostasis; Maret, W., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 85–127. ISBN 978-90-481-5916-1. [Google Scholar]
- Braconnier, B.; Páez, C.A.; Lambert, S.; Alié, C.; Henrist, C.; Poelman, D.; Pirard, J.-P.; Cloots, R.; Heinrichs, B. Ag-and SiO2-doped porous TiO2 with enhanced thermal stability. Microporous Mesoporous Mat. 2009, 122, 247–254. [Google Scholar] [CrossRef]
- Páez, C.A.; Poelman, D.; Pirard, J.-P.; Heinrichs, B. Unpredictable photocatalytic ability of H2-reduced rutile-TiO2 xerogel in the degradation of dye-pollutants under UV and visible light irradiation. Appl. Catal. B-Environ. 2010, 94, 263–271. [Google Scholar] [CrossRef]
- Association of Official Seed Analysts (AOSA). Rules for Testing Seeds. J. Seed Technol. 1993, 16, 1–113. [Google Scholar]
- SAS/STAT User’s Guide. Version 8. Available online: http://www.okstate.edu/sas/v8/saspdf/stat/pdfidx.htm (accessed on 7 February 2018).
- Zak, A.K.; Abrishami, M.E.; Majid, W.A.; Yousefi, R.; Hosseini, S. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fis. 2007, 53, 18–22. [Google Scholar]
- Ashar, A.; Iqbal, M.; Bhatti, I.A.; Ahmad, M.Z.; Qureshi, K.; Nisar, J.; Bukhari, I.H. Synthesis, characterization and photocatalytic activity of ZnO flower and pseudo-sphere: Nonylphenol ethoxylate degradation under UV and solar irradiation. J. Alloy. Compd. 2016, 678, 126–136. [Google Scholar] [CrossRef]
- Alkauskas, A.; Pasquarello, A. Band-edge problem in the theoretical determination of defect energy levels: The O vacancy in ZnO as a benchmark case. Phys. Rev. B 2011, 84, 125206. [Google Scholar] [CrossRef]
- Irimpan, L.; Nampoori, V.; Radhakrishnan, P.; Deepthy, A.; Krishnan, B. Size dependent fluorescence spectroscopy of nanocolloids of ZnO. J. Appl. Phys. 2007, 102, 063524. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.; Seager, C.; Tallant, D.; Voigt, J.; Gnade, B. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Brayner, R.; Dahoumane, S.A.; Yéprémian, C.; Djediat, C.; Meyer, M.; Couté, A.; Fiévet, F. ZnO nanoparticles: Synthesis, characterization, and ecotoxicological studies. Langmuir 2010, 26, 6522–6528. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, F.; Arshi, N.; Chaman, M.; Naqvi, A. Formation and characterization of ZnO nanopowder synthesized by sol–gel method. J. Alloy. Compd. 2010, 496, 399–402. [Google Scholar] [CrossRef]
- Wagner, G.; Korenkov, V.; Judy, J.D.; Bertsch, P.M. Nanoparticles composed of Zn and ZnO inhibit Peronospora tabacina spore germination in vitro and P. tabacina infectivity on tobacco leaves. Nanomaterials 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, T.; Kundu, S.; Rao, A.S. Zinc delivery to plants through seed coating with nano-zinc oxide particles. J. Plant Nutr. 2016, 39, 136–146. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Tu, C.; Hu, X.; Li, L.; Luo, Y.; Christie, P. Phytotoxicity of ZnO nanoparticles and the released Zn(II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ. Sci. Pollut. Res. 2015, 22, 11109–11117. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Bettger, W.J.; O’Dell, B.L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981, 28, 1425–1438. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990, 29, 5647–5659. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Tetsumura, T.; De Rybel, B.; dit Frey, N.F.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007, 134, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.M. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Morales, M.I.; Rico, C.M.; Hernandez-Viezcas, J.A.; Nunez, J.E.; Barrios, A.C.; Tafoya, A.; Flores-Marges, J.P.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J. Agric. Food Chem. 2013, 61, 6224–6230. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.; Sajanlal, P.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]






| Treatment | Germination (%) | Contamination (%) | ||
|---|---|---|---|---|
| SG | AA | SG | AA | |
| Uncoated | 80 ± 3 a | 68 ± 4 a | 70 ± 4 a | 45 ± 5 a |
| Starch-coated | 83 ± 2 a | 80 ± 2 b | 58 ± 3 ab | 40 ± 4 a |
| ZnO NPs | 97 ± 2 b | 90 ± 3 c | 37 ± 4 c | 8 ± 2 b |
| Treatment | Length (cm) | Diameter (mm) | |||
|---|---|---|---|---|---|
| SG | AA | SG | AA | ||
| Shoot | Uncoated | 12.8 ± 0.4 a | 8.1 ± 0.5 a | 2.8 ± 0.1 a | 1.6 ± 0.1 a |
| Starch-coated | 13.5 ± 0.5 ab | 9.6 ± 0.5 a | 3.1 ± 0.1 b | 1.7 ± 0.2 a | |
| ZnO NPs | 14.4 ± 0.5 b | 11.1 ± 0.6 b | 3.4 ± 0.1 c | 2.0 ± 0.1 b | |
| Length (cm) | Secondary Roots (Number) | ||||
| Root | Uncoated | 16.2 ± 0.7 a | 14.9 ± 0.6 a | 2.6 ± 0.1 a | 1.6 ± 0.1 a |
| Starch-coated | 20.3 ± 0.7 b | 16.6 ± 0.5 b | 3.3 ± 0.2 b | 2.9 ± 0.2 b | |
| ZnO NPs | 20.4 ± 0.7 b | 17.9 ± 0.6 b | 3.4 ± 0.2 b | 3.0 ± 0.2 b | |
| Band | Wavenumber (cm−1) | Functional Group and Commonly Assigned Component | |||||
|---|---|---|---|---|---|---|---|
| Shoots | Roots | ||||||
| Uncoated | Starch-Coated | ZnO NPs | Uncoated | Starch-Coated | ZnO NPs | ||
| A | 3127 | 3189 | 3244 | 3168 | 3190 | 3253 | N–H stretching vibrations (peptide and protein). |
| B | - | 2915 | 2920 | 2926 | 2926 | 2920 | C–H symmetric/asymmetric stretch (lipid). |
| C | 2359 | 2354 | 2366 | 2337 | 2343 | 2341 | N≡N stretch in primary amines. |
| D | - | 1627 | 1630 | 1579 | 1579 | 1582 | Aromatic C=C stretch (lignin). |
| E | - | - | 1410 | 1375 | 1364 | 1367 | C–H bends from symmetric –(CH3)n– –(CH2)n– (lipid, polysaccharide and cellulose). |
| F | - | - | 1243 | - | 1250 | 1249 | C–O–H deformation asymmetric (hemicellulose and cellulose). |
| G | - | 1036 | 1032 | 1033 | 1034 | 1037 | C–O stretching/C–O bending of the C–O–H carbohydrate. |
| Band | Band Area (Area Units) | |||||
|---|---|---|---|---|---|---|
| Shoots | Roots | |||||
| Uncoated | Starch-Coated | ZnO NPs | Uncoated | Starch-Coated | ZnO NPs | |
| A | 103.74 | 302.16 | 636.94 | 249.2 | 313 | 512.75 |
| B | 0 | 17.3 | 107.53 | 9.87 | 8.79 | 36.65 |
| C | 172.35 | 138.28 | 151.72 | 202.94 | 209.46 | 229.93 |
| D | 87.73 | 146.52 | 349.66 | 89.51 | 102.36 | 136.33 |
| E | 0 | 9.79 | 124.54 | 14.42 | 23.52 | 90.3 |
| F | 0 | 0 | 43.37 | 0 | 8.25 | 20.47 |
| G | 21.16 | 384.55 | 1139.9 | 308.41 | 466 | 714.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Urbina, J.; Cruz-Alonso, A.; Santander-González, M.; Méndez-Albores, A.; Vázquez-Durán, A. Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize. Nanomaterials 2018, 8, 247. https://doi.org/10.3390/nano8040247
Estrada-Urbina J, Cruz-Alonso A, Santander-González M, Méndez-Albores A, Vázquez-Durán A. Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize. Nanomaterials. 2018; 8(4):247. https://doi.org/10.3390/nano8040247
Chicago/Turabian StyleEstrada-Urbina, Juan, Alejandro Cruz-Alonso, Martha Santander-González, Abraham Méndez-Albores, and Alma Vázquez-Durán. 2018. "Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize" Nanomaterials 8, no. 4: 247. https://doi.org/10.3390/nano8040247
APA StyleEstrada-Urbina, J., Cruz-Alonso, A., Santander-González, M., Méndez-Albores, A., & Vázquez-Durán, A. (2018). Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize. Nanomaterials, 8(4), 247. https://doi.org/10.3390/nano8040247