Non-Canonical Activation of the Epidermal Growth Factor Receptor by Carbon Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Particle Characterization
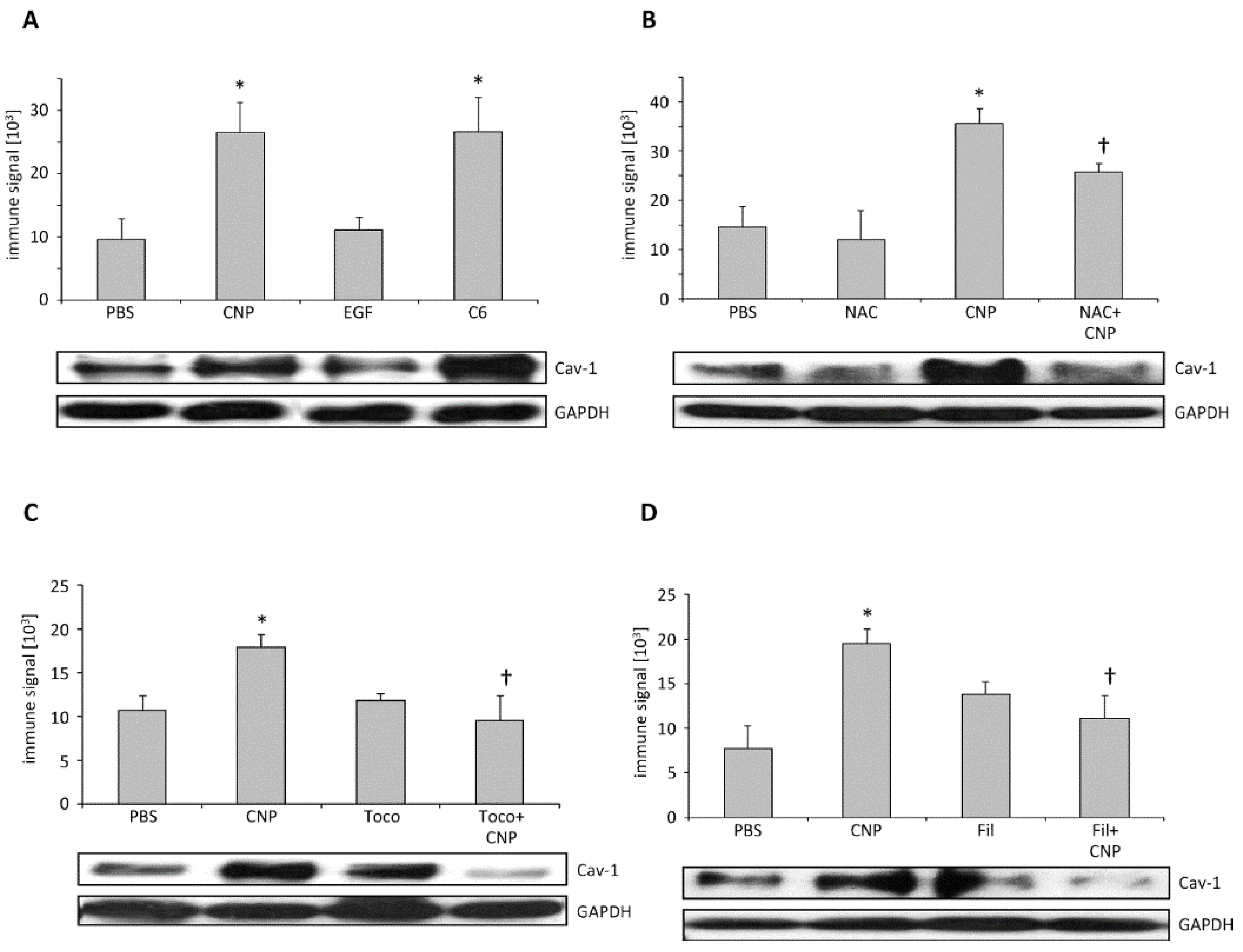
2.2. Caveolin-1 Is Involved in EGFR Activation after Carbon Nanoparticle Exposure
2.3. Carbon Nanoparticles Induce Higher Order Structures of Caveolin-1
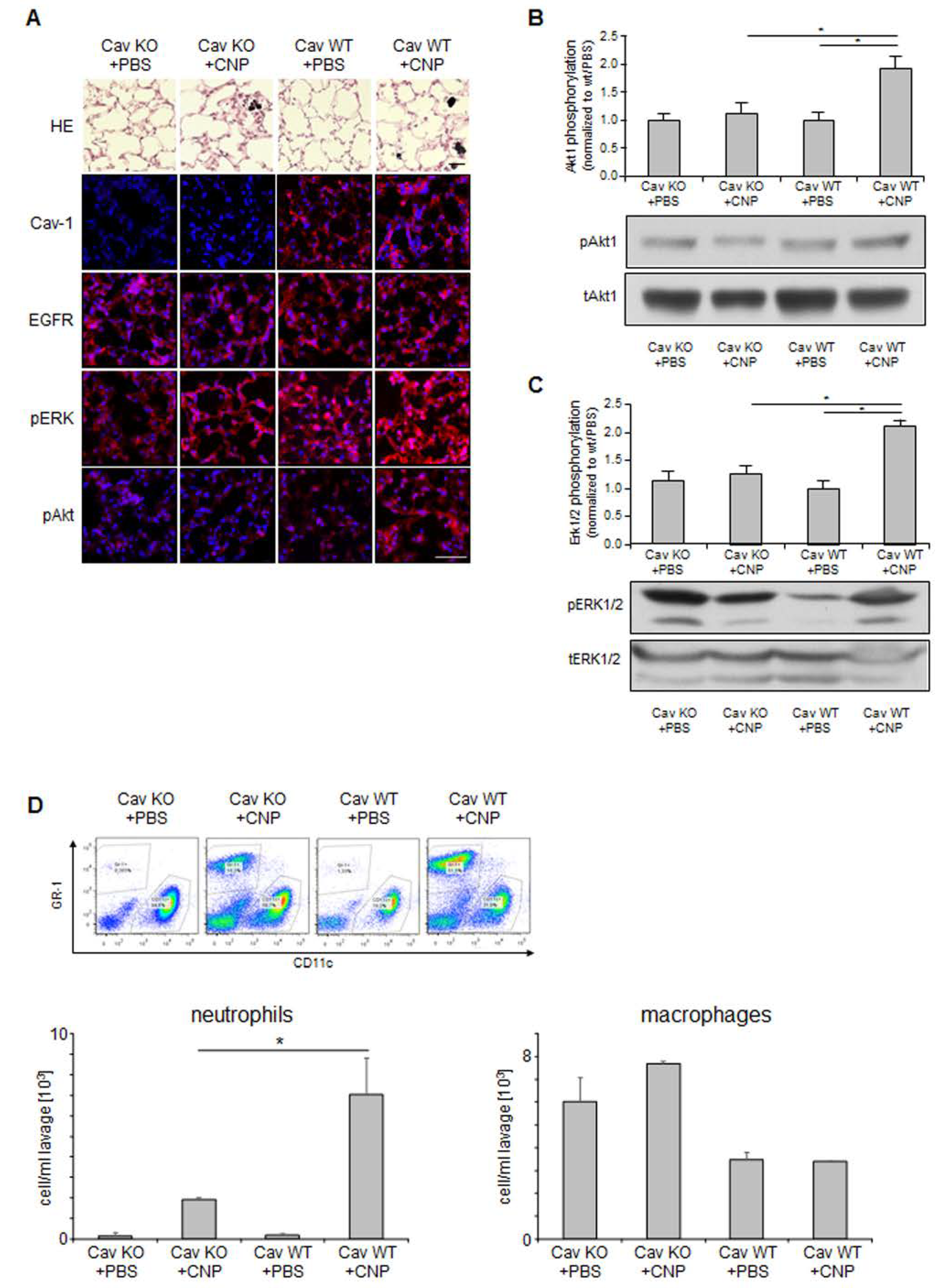
2.4. Non-Canonical EGFR Activation In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Exposure
4.3. Protein Isolation
4.4. Protein Analyses
4.5. Immunostaining
4.6. Animal Experiments
4.7. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Comfort, K.K.; Maurer, E.I.; Braydich-Stolle, L.K.; Hussain, S.M. Interference of silver, gold, and iron oxide nanoparticles on epidermal growth factor signal transduction in epithelial cells. ACS Nano 2011, 5, 10000–10008. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, J.; Isono, K.; Takeyama, K.; Tagaya, E.; Nakata, J.; Nagai, A. Ultrafine carbon black particles stimulate proliferation of human airway epithelium via egf receptor-mediated signaling pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1127–L1133. [Google Scholar] [CrossRef] [PubMed]
- Sydlik, U.; Bierhals, K.; Soufi, M.; Abel, J.; Schins, R.P.; Unfried, K. Ultrafine carbon particles induce apoptosis and proliferation in rat lung epithelial cells via specific signaling pathways both using egf-r. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L725–L733. [Google Scholar] [CrossRef] [PubMed]
- Unfried, K.; Albrecht, C.; Klotz, L.-O.; Von Mikecz, A.; Grether-Beck, S.; Schins, R.P.F. Cellular responses to nanoparticles: Target structures and mechanisms. Nanotoxicology 2007, 1, 52–71. [Google Scholar] [CrossRef]
- Ovrevik, J.; Refsnes, M.; Totlandsdal, A.I.; Holme, J.A.; Schwarze, P.E.; Lag, M. TACE/TGF-alpha/EGFR regulates CXCL8 in bronchial epithelial cells exposed to particulate matter components. Eur. Respir. J. 2011, 38, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Skuland, T.; Ovrevik, J.; Lag, M.; Schwarze, P.; Refsnes, M. Silica nanoparticles induce cytokine responses in lung epithelial cells through activation of a p38/TACE/TGF-alpha/EGFR-pathway and NF-kappabeta signalling. Toxicol. Appl. Pharmacol. 2014, 279, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Goldkorn, T.; Balaban, N.; Matsukuma, K.; Chea, V.; Gould, R.; Last, J.; Chan, C.; Chavez, C. EGF-receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am. J. Respir. Cell Mol. Biol. 1998, 19, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Buchner, N.; Ale-Agha, N.; Jakob, S.; Sydlik, U.; Kunze, K.; Unfried, K.; Altschmied, J.; Haendeler, J. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp. Gerontol. 2013, 48, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Peuschel, H.; Sydlik, U.; Grether-Beck, S.; Felsner, I.; Stockmann, D.; Jakob, S.; Kroker, M.; Haendeler, J.; Gotic, M.; Bieschke, C.; et al. Carbon nanoparticles induce ceramide- and lipid raft-dependent signalling in lung epithelial cells: A target for a preventive strategy against environmentally-induced lung inflammation. Part. Fibre Toxicol. 2012, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Weissenberg, A.; Sydlik, U.; Peuschel, H.; Schroeder, P.; Schneider, M.; Schins, R.P.; Abel, J.; Unfried, K. Reactive oxygen species as mediators of membrane-dependent signaling induced by ultrafine particles. Free Radic. Biol. Med. 2010, 49, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Unfried, K.; Sydlik, U.; Bierhals, K.; Weissenberg, A.; Abel, J. Carbon nanoparticle-induced lung epithelial cell proliferation is mediated by receptor-dependent akt activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L358–L367. [Google Scholar] [CrossRef] [PubMed]
- Peuschel, H.; Sydlik, U.; Haendeler, J.; Buchner, N.; Stockmann, D.; Kroker, M.; Wirth, R.; Brock, W.; Unfried, K. C-SRC-mediated activation of ERK1/2 is a reaction of epithelial cells to carbon nanoparticle treatment and may be a target for a molecular preventive strategy. Biol. Chem. 2010, 391, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Haussinger, D.; Oesterhelt, F. Effect of the compatible solute ectoine on the stability of the membrane proteins. Protein Pept. Lett. 2012, 19, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Sydlik, U.; Gallitz, I.; Albrecht, C.; Abel, J.; Krutmann, J.; Unfried, K. The compatible solute ectoine protects against nanoparticle-induced neutrophilic lung inflammation. Am. J. Respir. Crit. Care Med. 2009, 180, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Filosto, S.; Khan, E.M.; Tognon, E.; Becker, C.; Ashfaq, M.; Ravid, T.; Goldkorn, T. Egf receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS ONE 2011, 6, e23240. [Google Scholar] [CrossRef] [PubMed]
- Goldkorn, T.; Filosto, S.; Chung, S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid. Redox Signal. 2014, 21, 2149–2174. [Google Scholar] [CrossRef] [PubMed]
- Balbis, A.; Parmar, A.; Wang, Y.; Baquiran, G.; Posner, B.I. Compartmentalization of signaling-competent epidermal growth factor receptors in endosomes. Endocrinology 2007, 148, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Sun, P.; Paller, A.S. Ganglioside induces caveolin-1 redistribution and interaction with the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 47028–47034. [Google Scholar] [CrossRef] [PubMed]
- Kroker, M.; Sydlik, U.; Autengruber, A.; Cavelius, C.; Weighardt, H.; Kraegeloh, A.; Unfried, K. Preventing carbon nanoparticle-induced lung inflammation reduces antigen-specific sensitization and subsequent allergic reactions in a mouse model. Part. Fibre Toxicol. 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, K.E.; Carter, J.M.; Iype, P.T.; Kumari, H.L.; Crosby, L.L.; Aardema, M.J.; Isfort, R.J.; Cody, D.; Chestnut, M.H.; Burns, J.L.; et al. Establishment of immortalized alveolar type II epithelial cell lines from adult rats. Cell. Dev. Biol. Anim. 1995, 31, 516–527. [Google Scholar] [CrossRef]
- Mineo, C.; Gill, G.N.; Anderson, R.G. Regulated migration of epidermal growth factor receptor from caveolae. J. Biol. Chem. 1999, 274, 30636–30643. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Graves, L.M.; Gill, G.N.; Parsons, S.J.; Samet, J.M. Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced ras activation. J. Biol. Chem. 2002, 277, 24252–24257. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wages, P.A.; Devlin, R.B.; Diaz-Sanchez, D.; Peden, D.B.; Samet, J.M. SRC-mediated EGF receptor activation regulates ozone-induced interleukin 8 expression in human bronchial epithelial cells. Environ. Health Perspect. 2015, 123, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.L.; Powell, K.; Madden, J.M.; Eblen, S.T.; Boerner, J.L. EGFR tyrosine 845 phosphorylation-dependent proliferation and transformation of breast cancer cells require activation of p38 mapk. Transl. Oncol. 2012, 5, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sargiacomo, M.; Scherer, P.E.; Tang, Z.; Kubler, E.; Song, K.S.; Sanders, M.C.; Lisanti, M.P. Oligomeric structure of caveolin: Implications for caveolae membrane organization. Proc. Natl. Acad. Sci. USA 1995, 92, 9407–9411. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Nichols, B.J. Exploring the caves: Cavins, caveolins and caveolae. Trends Cell Biol. 2010, 20, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Howard, G.; Mendoza-Topaz, C.; Deerinck, T.; Mackey, M.; Sandin, S.; Ellisman, M.H.; Nichols, B.J. Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biol. 2013, 11, e1001640. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Reed, W.; Lenz, A.G.; Jaspers, I.; Silbajoris, R.; Nick, H.S.; Samet, J.M. Ultrafine carbon particles induce interleukin-8 gene transcription and p38 mapk activation in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L432–L441. [Google Scholar] [CrossRef] [PubMed]
- Autengruber, A.; Sydlik, U.; Kroker, M.; Hornstein, T.; Ale-Agha, N.; Stockmann, D.; Bilstein, A.; Albrecht, C.; Paunel-Gorgulu, A.; Suschek, C.V.; et al. Signalling-dependent adverse health effects of carbon nanoparticles are prevented by the compatible solute mannosylglycerate (firoin) in vitro and in vivo. PLoS ONE 2014, 9, e111485. [Google Scholar] [CrossRef] [PubMed]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yin, R.; Mutze, K.; Yu, Y.; Takenaka, S.; Konigshoff, M.; Stoeger, T. No involvement of alveolar macrophages in the initiation of carbon nanoparticle induced acute lung inflammation in mice. Part. Fibre Toxicol. 2016, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ohoka, A.; Kolmodin, N.J.; Pan, D. Next generation carbon nanoparticles for efficient gene therapy. Mol. Pharm. 2015, 12, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Modugno, G.; Menard-Moyon, C.; Prato, M.; Bianco, A. Carbon nanomaterials combined with metal nanoparticles for theranostic applications. Br. J. Pharmacol. 2015, 172, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, S.; Zhang, Z.; He, J.; Xiao, S.; Fan, P.; Xie, J.; Gu, X.; Li, Y.; Zheng, W. Evaluation of the clinical value of carbon nanoparticles as lymph node tracer in differentiated thyroid carcinoma requiring reoperation. Int. J. Clin. Oncol. 2016, 21, 68–74. [Google Scholar]
- Huynh, E.; Li, J. EGF and EGFR: Promising targets for modulating inflammation and mucosal healing therapy in IBD. Inflamm. Cell Signal. 2015, 2. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSS) to release growth factors for wound healing and skin regeneration. Nanomedicine 2015, 11, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, L.; Grandal, M.V.; Knudsen, S.L.; van Deurs, B.; Grovdal, L.M. Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS ONE 2013, 8, e58148. [Google Scholar] [CrossRef] [PubMed]
- Berken, A.; Abel, J.; Unfried, K. Beta1-integrin mediates asbestos-induced phosphorylation of AKT and ERK1/2 in a rat pleural mesothelial cell line. Oncogene 2003, 22, 8524–8528. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stöckmann, D.; Spannbrucker, T.; Ale-Agha, N.; Jakobs, P.; Goy, C.; Dyballa-Rukes, N.; Hornstein, T.; Kümper, A.; Kraegeloh, A.; Haendeler, J.; et al. Non-Canonical Activation of the Epidermal Growth Factor Receptor by Carbon Nanoparticles. Nanomaterials 2018, 8, 267. https://doi.org/10.3390/nano8040267
Stöckmann D, Spannbrucker T, Ale-Agha N, Jakobs P, Goy C, Dyballa-Rukes N, Hornstein T, Kümper A, Kraegeloh A, Haendeler J, et al. Non-Canonical Activation of the Epidermal Growth Factor Receptor by Carbon Nanoparticles. Nanomaterials. 2018; 8(4):267. https://doi.org/10.3390/nano8040267
Chicago/Turabian StyleStöckmann, Daniel, Tim Spannbrucker, Niloofar Ale-Agha, Philipp Jakobs, Christine Goy, Nadine Dyballa-Rukes, Tamara Hornstein, Alexander Kümper, Annette Kraegeloh, Judith Haendeler, and et al. 2018. "Non-Canonical Activation of the Epidermal Growth Factor Receptor by Carbon Nanoparticles" Nanomaterials 8, no. 4: 267. https://doi.org/10.3390/nano8040267



_Haendeler.png)

