Carbon-Based Fe3O4 Nanocomposites Derived from Waste Pomelo Peels for Magnetic Solid-Phase Extraction of 11 Triazole Fungicides in Fruit Samples
Abstract
:1. Introduction
2. Results and Discussion
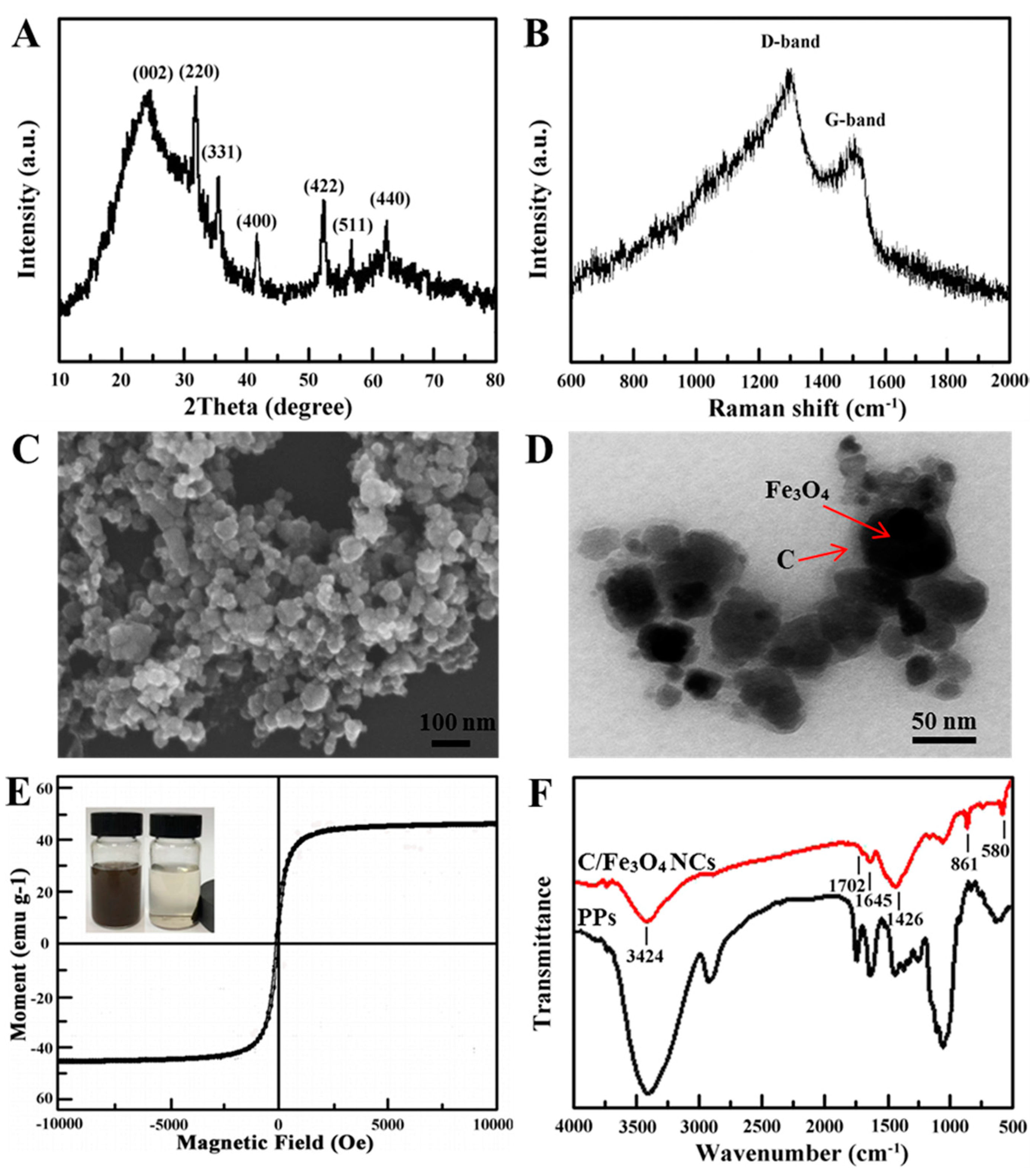
2.1. Characterization of C/Fe3O4 NCs
2.2. MSPE Optimization
2.2.1. Effect of Activation Factor
2.2.2. Effect of Extraction Time and Adsorbent Amount
2.2.3. Effect of pH
2.2.4. Effect of Salinity
2.2.5. Effect of Desorption Agent Type
2.2.6. Effect of Desorption Solvent Volume and Desorption Time
2.3. Validation of the Method
2.4. Reusability of C/Fe3O4 NCs
2.5. Analysis of Real Samples
2.6. Comparison with Other Methods
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of C/Fe3O4 NCs
3.4. Sample Preparation and MSPE Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.B.; Dong, F.S.; Liu, X.G.; Xu, J.; Li, J.; Kong, Z.Q.; Chen, X.; Liang, X.Y.; Zheng, Y.Q. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1224, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Belda, M.; Garrido, I.; Campillo, N.; Viñas, P.; Hellín, P.; Flores, P.; Fenoll, J. Combination of solvent extractants for dispersive liquid-liquid microextraction of fungicides from water and fruit samples by liquid chromatography with tandem mass spectrometry. Food Chem. 2017, 233, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of six fungicides in greenhouse-grown tomatoes with processing and health risk. Environ. Sci. Pollut. Res. 2016, 23, 11885–11900. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, S.; Dehouck, P. Review of analytical methods for the determination of pesticide residues in grapes. J. Chromatogr. A 2016, 1433, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nodeh, H.R.; Sereshti, H.; Gaikani, H.; Kamboh, M.A.; Afsharsaveh, Z. Magnetic graphene coated inorganic-organic hybrid nanocomposite for enhanced preconcentration of selected pesticides in tomato and grape. J. Chromatogr. A 2017, 1509, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Santos, M.G.; Figueiredo, E.C. Solid-phase extraction of triazole fungicides from water samples using disks impregnated with carbon nanotubes followed by GC-MS analysis. Int. J. Environ. Anal. Chem. 2017, 97, 29–41. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.P. Magnetic graphene solid-phase extraction for the determination of carbamate pesticides in tomatoes coupled with high performance liquid chromatography. Talanta 2015, 141, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TRAC Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z.Q. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, R.Y.; Xiao, R.B.; Wu, Q.H.; Wang, C.; Wang, Z. Preparation of a magnetic porous carbon with hierarchical structures from waste biomass for the extraction of some carbamates. J. Sep. Sci. 2017, 40, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, L.; Zhang, Z.H.; Wu, Q.H.; Wang, Z. Magnetic Biomass Activated Carbon-Based Solid-Phase Extraction Coupled with High Performance Liquid Chromatography for the Determination of Phenylurea Herbicides in Bottled Rose Juice and Water Samples. Food Anal. Meth. 2016, 9, 80–87. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.X.; You, L.J.; Shen, X.Q.; Li, S.J. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2017, 241, 285–292. [Google Scholar] [CrossRef]
- Li, H.Z.; Sun, Z.B.; Zhang, L.; Tian, Y.X.; Cui, G.J.; Yan, S.Q. A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloid Surf. A Physicochem. Eng. Asp. 2016, 489, 191–199. [Google Scholar] [CrossRef]
- Zhu, J.H.; Liu, Q.; Li, Z.S.; Liu, J.Y.; Zhang, H.S.; Li, R.M.; Wang, J.; Emelchenko, G.A. Recovery of uranium (VI) from aqueous solutions using a modified honeycomb-like porous carbon material. Dalton Trans. 2017, 46, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.H.; Ye, L.; Huang, Z.H.; Xu, Q.; Bai, Y.; Kang, F.Y.; Yang, Q.H. A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 2014, 6, 13831–13837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z.Q. Citrus pectin derived ultrasmall Fe3O4@C nanoparticles as a high-performance adsorbent toward removal of methylene blue. J. Mol. Liq. 2016, 222, 995–1002. [Google Scholar] [CrossRef]
- Li, Y.; Leng, T.H.; Lin, H.Q.; Lin, H.; Deng, C.H.; Xu, X.Q.; Yao, N.; Yang, P.Y.; Zhang, X.M. Preparation of Fe3O4@ ZrO2 core-shell microspheres as affinity probes for selective enrichment and direct determination of phosphopeptides using matrix-assisted laser desorption ionization mass spectrometry. J. Proteome Res. 2007, 6, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Niu, H.Y.; Hu, Z.J.; Cai, Y.Q.; Shi, Y.L. Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2010, 1217, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhou, Z.Q. Citrus pectin-derived crbon microspheres with superior adsorption ability for methylene blue. Nanomaterials 2017, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.M.; Allam, O.A.; Khairy, M.; Mohammed, K.M.; Elkhatib, R.M.; Hamed, M.A. High surface area nanostructured activated carbons derived from sustainable sorghum stalk. J. Mol. Liq. 2017, 247, 386–396. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Simal-Gándara, J. Multiresidue determination of 11 new fungicides in grapes and wines by liquid–liquid extraction/clean-up and programmable temperature vaporization injection with analyte protectants/gas chromatography/ion trap mass spectrometry. J. Chromatogr. A 2009, 1216, 6033–6042. [Google Scholar] [CrossRef] [PubMed]
- Mahpishanian, S.; Sereshti, H. One-step green synthesis of β-cyclodextrin/iron oxide-reduced graphene oxide nanocomposite with high supramolecular recognition capability: Application for vortex-assisted magnetic solid phase extraction of organochlorine pesticides residue from honey samples. J. Chromatogr. A 2017, 1485, 32–43. [Google Scholar] [PubMed]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, H. Pyrethroid residue determination in organic and conventional vegetables using liquid-solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem. 2017, 217, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zang, X.H.; Chang, Q.Y.; Zhang, G.J.; Wang, C.; Wang, Z. Determination of Triazole Fungicides in Vegetable Samples, by Magnetic Solid-Phase Extraction with Graphene-Coated Magnetic Nanocomposite as Adsorbent Followed by Gas Chromatography-Mass Spectrometry Detection. Food Anal. Meth. 2014, 7, 318–325. [Google Scholar] [CrossRef]
- Chen, F.J.; Song, Z.Y.; Nie, J.; Yu, G.W.; Li, Z.G.; Lee, M. Ionic liquid-based carbon nanotube coated magnetic nanoparticles as adsorbent for the magnetic solid phase extraction of triazole fungicides from environmental water. RSC Adv. 2016, 6, 81877–81885. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, X.C.; Zhao, P.F.; Sun, H.; Ye, X.; Liang, N.; Zhao, L.S. Trace determination of five triazole fungicide residues in traditional Chinese medicine samples by dispersive solid-phase extraction combined with ultrasound-assisted dispersive liquid–liquid microextraction and UHPLC–MS/MS. J. Sep. Sci. 2017, 40, 3257–3266. [Google Scholar] [CrossRef] [PubMed]



| Compounds | Rt (min) | Quantifier and Qualifier (m/z) | Regression Equations | R2 | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|
| Triadimefon | 15.395 | 208, 81, 210 | y = 30.26x + 1262 | 0.9916 | 0.15 | 0.50 |
| Triadimenol | 16.586 | 112, 168, 130, | y = 34.65x + 2031 | 0.9990 | 0.26 | 0.88 |
| Triflumizole | 16.785 | 206, 179, 186 | y = 13.99x + 464 | 0.9997 | 0.32 | 1.08 |
| Hexaconazole | 17.467 | 214, 231, 256 | y = 17.15x + 907.2 | 0.9951 | 0.26 | 0.86 |
| Flusilazole | 18.005 | 233, 315, 206 | y = 89.79x + 2170 | 0.9970 | 0.12 | 0.39 |
| Diniconazole | 18.600 | 268, 270, 232 | y = 39.38x − 527.5 | 0.9947 | 0.14 | 0.46 |
| Epoxiconazole | 19.395 20.019 | 192, 183, 138 | y = 19.39x + 340.5 | 0.9930 | 0.55 | 1.85 |
| Propiconazole | 19.403 19.539 | 259, 171, 261 | y = 42.2x + 413.4 | 0.9999 | 0.13 | 0.45 |
| Tebuconazole | 19.866 | 250, 252, 163 | y = 31.35x + 474 | 0.9984 | 0.16 | 0.54 |
| Bitertanol | 22.406 | 170, 112, 141 | y = 76.21x + 1352 | 0.9951 | 0.18 | 0.58 |
| Difenoconazole | 25.309 | 323, 325, 265 | y = 29.65x + 779.4 | 0.9982 | 0.15 | 0.51 |
| Compounds | Spiked Level (μg/kg) | Intra-Day (n = 6) | Inter-Day (n = 6) | ||
|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Triadimefon | 10 | 87.6 | 5.4 | 85.6 | 6.7 |
| 20 | 91.2 | 3.6 | 89.6 | 4.4 | |
| 50 | 96.8 | 2.3 | 93.3 | 4.6 | |
| Triadimenol | 10 | 90.6 | 4.2 | 87.7 | 5.9 |
| 20 | 92.5 | 4.1 | 88.6 | 4.7 | |
| 50 | 104.3 | 2.6 | 97.1 | 3.5 | |
| Triflumizole | 10 | 109.9 | 5.2 | 102.8 | 6.5 |
| 20 | 104.2 | 3.2 | 97.2 | 6.1 | |
| 50 | 97.8 | 2.1 | 98.1 | 3.8 | |
| Hexaconazole | 10 | 98.2 | 4.9 | 95.1 | 7.6 |
| 20 | 99.2 | 3.2 | 95.9 | 4.9 | |
| 50 | 103.5 | 2.6 | 97.7 | 3.8 | |
| Flusilazole | 10 | 108.7 | 6.6 | 104.0 | 8.4 |
| 20 | 98.5 | 4.2 | 95.3 | 6.4 | |
| 50 | 99.2 | 3.2 | 96.6 | 4.5 | |
| Diniconazole | 10 | 103.1 | 4.7 | 96.4 | 7.9 |
| 20 | 101.0 | 3.8 | 98.5 | 4.6 | |
| 50 | 102.4 | 2.8 | 97.7 | 3.7 | |
| Epoxiconazole | 10 | 103.8 | 5.4 | 98.4 | 5.7 |
| 20 | 99.1 | 3.9 | 96.9 | 4.8 | |
| 50 | 101.5 | 3.2 | 99.4 | 3.6 | |
| Propiconazole | 10 | 85.7 | 4.6 | 83.5 | 6.0 |
| 20 | 92.5 | 4.3 | 86.1 | 5.6 | |
| 50 | 95.4 | 3.6 | 89.4 | 4.2 | |
| Tebuconazole | 10 | 83.9 | 6.1 | 82.1 | 6.9 |
| 20 | 90.0 | 5.2 | 86.1 | 7.0 | |
| 50 | 96.3 | 3.4 | 95.0 | 4.2 | |
| Bitertanol | 10 | 107.8 | 6.2 | 98.1 | 7.3 |
| 20 | 96.2 | 5.1 | 94.3 | 5.4 | |
| 50 | 102.1 | 2.5 | 100.7 | 4.6 | |
| Difenoconazole | 10 | 89.0 | 5.0 | 84.6 | 5.3 |
| 20 | 92.6 | 4.2 | 90.3 | 4.8 | |
| 50 | 93.9 | 2.9 | 92.3 | 4.2 | |
| Compounds | Apple | Pear | Orange | Banana | Peach |
|---|---|---|---|---|---|
| Triadimefon | ND | 0.41 ± 0.03 | ND | ND | ND |
| Triadimenol | ND | ND | ND | ND | ND |
| Triflumizole | ND | ND | ND | ND | ND |
| Hexaconazole | 0.52 ± 0.03 | 0.27 ± 0.07 | ND | ND | 0.49 ± 0.03 |
| Flusilazole | 0.19 ± 0.05 | ND | ND | ND | 0.33 ± 0.01 |
| Diniconazole | ND | ND | ND | ND | ND |
| Epoxiconazole | ND | ND | ND | ND | ND |
| Propiconazole | ND | ND | ND | ND | ND |
| Tebuconazole | ND | ND | ND | ND | ND |
| Bitertanol | 0.20 ± 0.04 | ND | ND | ND | ND |
| Difenoconazole | ND | ND | ND | ND | ND |
| Adsorbent | Analyte Number | Sample | Determination | LOD (μg/kg) | RSD (%) | Extraction Time (min) | Ref. |
|---|---|---|---|---|---|---|---|
| CNTs | 3 | Water | GC-MS | 0.02–0.03 | <12 | >30 | [6] |
| G-Fe3O4 | 7 | Vegetables | GC-MS | 0.01–0.10 | <10.6 | 20 | [26] |
| IL-Fe3O4@MWCNTs | 6 | Water | GC-MS | 0.05–0.22 | <10.5 | 8 | [27] |
| GCB,C18 | 5 | Medicines | UPLC-MS/MS | 0.50–1.10 | <11.7 | >30 | [28] |
| C/Fe3O4 NCs | 11 | Fruits | GC-MS | 0.12–0.55 | <9.7 | 2 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Zhang, W.; Cao, S.; Wang, G.; Zhou, Z. Carbon-Based Fe3O4 Nanocomposites Derived from Waste Pomelo Peels for Magnetic Solid-Phase Extraction of 11 Triazole Fungicides in Fruit Samples. Nanomaterials 2018, 8, 302. https://doi.org/10.3390/nano8050302
Ren K, Zhang W, Cao S, Wang G, Zhou Z. Carbon-Based Fe3O4 Nanocomposites Derived from Waste Pomelo Peels for Magnetic Solid-Phase Extraction of 11 Triazole Fungicides in Fruit Samples. Nanomaterials. 2018; 8(5):302. https://doi.org/10.3390/nano8050302
Chicago/Turabian StyleRen, Keyu, Wenlin Zhang, Shurui Cao, Guomin Wang, and Zhiqin Zhou. 2018. "Carbon-Based Fe3O4 Nanocomposites Derived from Waste Pomelo Peels for Magnetic Solid-Phase Extraction of 11 Triazole Fungicides in Fruit Samples" Nanomaterials 8, no. 5: 302. https://doi.org/10.3390/nano8050302




