Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of BC Membranes; Defibrillation of BC Membranes to Obtain Nanocellulose
2.3. Plasma Treatment of NC
2.4. Characterization
3. Results and Discussion
3.1. NC Morphology before and after Plasma Treatments
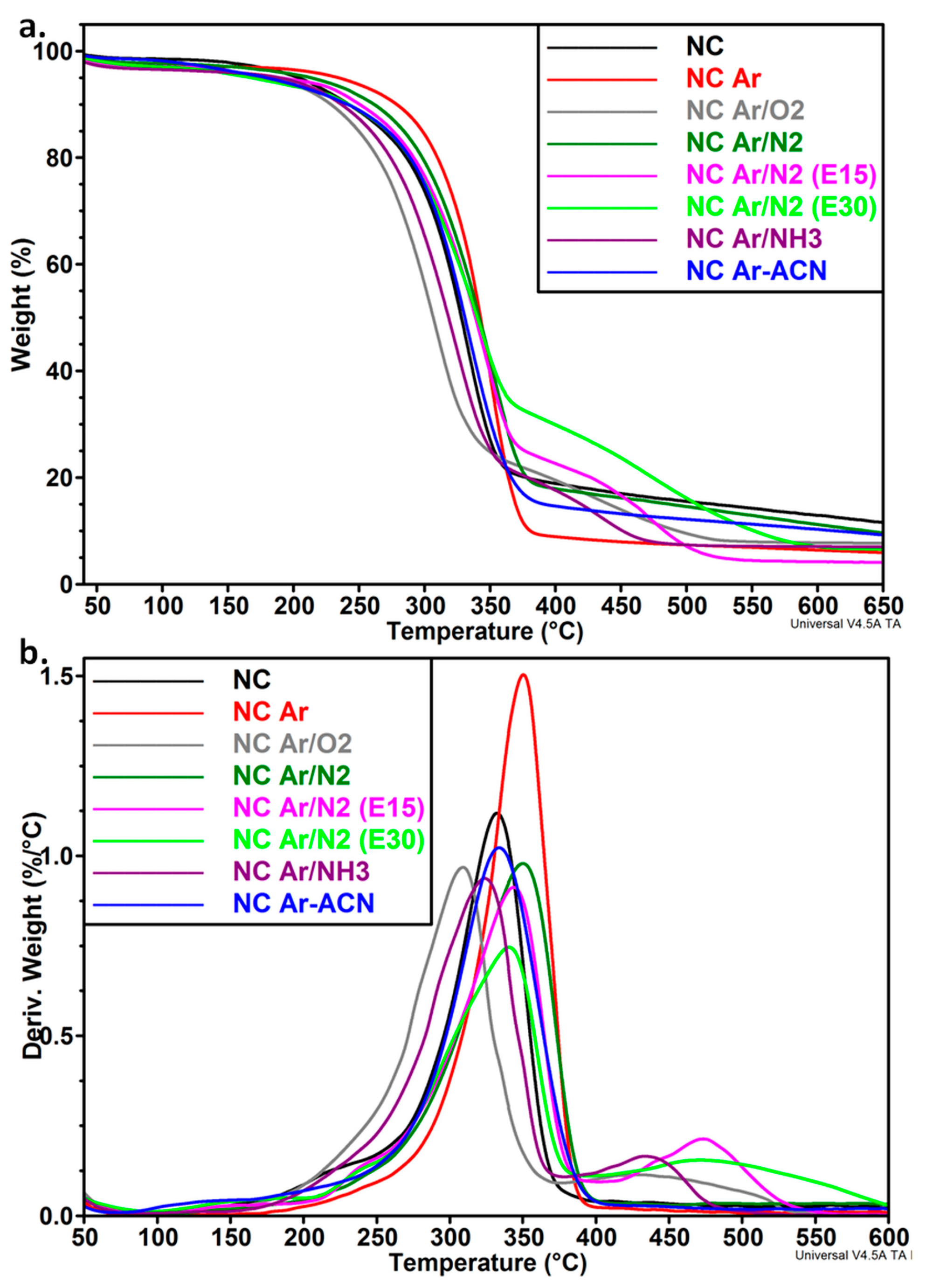
3.2. Thermal Analysis of NC before and after the Treatments
3.3. Surface Analysis by ATR-FTIR
3.4. Surface Analysis by XPS
3.5. Effect of Plasma Treated NC on PHB Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Sindhu, K.A.; Prasanth, R.; Thakur, V.K. Medical applications of cellulose and its derivatives: Present and future. In Nanocellulose Polymer Nanocomposites. Fundamentals and Applications; Thakur, V.K., Ed.; Wiley-Scrivener: Hoboken, NJ, USA, 2015; pp. 461–462. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Karimi, S.; Tahir, P.M.; Karimi, A.; Dufresne, A.; Abdulkhani, A. Kenaf bast cellulosic fibers hierarchy: A comprehensive approach from micro to nano. Carbohydr. Polym. 2014, 101, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Leao, R.M.; Mileo, P.C.; Maia, J.M.L.L.; Luz, S.M. Environmental and technical feasibility of cellulose nanocrystal manufacturing from sugarcane bagasse. Carbohydr. Polym. 2017, 175, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Chiulan, I.; Panaitescu, D.M.; Nicolae, C.A.; Ghiurea, M.; Galan, A.M. Isolation of cellulose nanocrystals from plum seed shells, structural and morphological characterization. Mater. Lett. 2017, 194, 160–163. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial nanocellulose from side-streams of Kombucha beverages production: Preparation and physical-chemical properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef]
- Martinez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of the nanofabrication by acid hydrolysis of bacterial cellulose nanowhiskers. Carbohydr. Polym. 2011, 85, 228–236. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Casarica, A.; Nicolae, C.A.; Ghiurea, M.; Trusca, R.; Damian, C.M. Structural and morphological characterization of bacterial cellulose nano-reinforcements prepared by mechanical route. Mater. Des. 2016, 110, 790–801. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K. Harvesting fibrils from bacterial cellulose pellicles and subsequent formation of biodegradable poly-3-hydroxybutyrate nanocomposites. Cellulose 2014, 21, 4299–4308. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Feitosa, J.P.A.; Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; Filho, M.M.S.; Rosa, M.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhang, S.; Sheng, L.; Liao, S.; Xi, T.; Zhang, Z. TEMPO-mediated oxidation of bacterial cellulose in a bromide-free system. Colloid Polym. Sci. 2013, 291, 2985–2992. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Hu, D.; Ren, K.; Yao, F.; Zhu, Y.; Gao, C.; Wan, Y. Characterization of TEMPO-oxidized bacterial cellulose scaffolds for tissue engineering applications. Mater. Chem. Phys. 2013, 143, 373–379. [Google Scholar] [CrossRef]
- Shao, W.; Wu, J.; Liu, H.; Ye, S.; Jiang, L.; Liu, X. Novel bioactive surface functionalization of bacterial cellulose membrane. Carbohydr. Polym. 2017, 178, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Phisalaphong, M.; Newby, B.Z. Modification of bacterial cellulose with organosilanes to improve attachment and spreading of human fibroblasts. Cellulose 2015, 22, 2311–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Rojo, U.M.; Cerrutti, P.; Vazquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics. Appl. Surf. Sci. 2017, 392, 1126–1133. [Google Scholar] [CrossRef]
- Kan, C.; Lam, C. Atmospheric pressure plasma treatment for grey cotton knitted fabric. Polymers 2018, 10, 53. [Google Scholar] [CrossRef]
- Prabhu, S.; Vaideki, K.; Anitha, S. Effect of microwave argon plasma on the glycosidic and hydrogen bonding system of cotton cellulose. Carbohydr. Polym. 2017, 156, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, L.; Abidi, N. Microwave plasma induced grafting of oleic acid on cotton fabric surfaces. Appl. Surf. Sci. 2012, 258, 4636–4641. [Google Scholar] [CrossRef]
- Samanta, K.K.; Joshi, A.G.; Jassal, M.; Agrawal, A.K. Study of hydrophobic finishing of cellulosic substrate using He/1,3-butadiene plasma at atmospheric pressure. Surf. Coat. Technol. 2012, 213, 65–76. [Google Scholar] [CrossRef]
- Calvimontes, A.; Mauersberger, P.; Nitschke, M.; Dutschk, V.; Simon, F. Effects of oxygen plasma on cellulose surface. Cellulose 2011, 18, 803–809. [Google Scholar] [CrossRef]
- Flynn, C.N.; Byrne, C.P.; Meenan, B.J. Surface modification of cellulose via atmospheric pressure plasma processing in air and ammonia–nitrogen gas. Surf. Coat. Technol. 2013, 233, 108–118. [Google Scholar] [CrossRef]
- Gaiolas, C.; Belgacem, M.N.; Silva, L.; Thielemans, W.; Costa, A.P.; Nunes, M.; Silva, M.J.S. Green chemicals and process to graft cellulose fibers. J. Colloid Interface Sci. 2009, 330, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.C.; Totolin, M.; Tibirna, C.M.; Sdrobis, A.; Stevanovic, T.; Vasile, C. Grafting of softwood kraft pulps fibers with fatty acids under cold plasma conditions. Int. J. Biol. Macromol. 2011, 48, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Bhanthumnavin, W.; Wanichapichart, P.; Taweepreeda, W.; Sirijarukula, S.; Paosawatyanyong, B. Surface modification of bacterial cellulose membrane by oxygen plasma treatment. Surf. Coat. Technol. 2016, 306, 272–278. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Andrade, F.K.; Alves, C., Jr.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Vander Wielen, L.C.; Ostenson, M.; Gatenholm, P.; Ragauskas, A.J. Surface modification of cellulosic fibers using dielectric-barrier discharge. Carbohydr. Polym. 2006, 65, 179–184. [Google Scholar] [CrossRef]
- Relvas, C.; Castro, G.; Rana, S.; Fangueiro, R. Characterization of physical, mechanical and chemical properties of Quiscal fibres: The influence of atmospheric DBD plasma treatment. Plasma Chem. Plasma Process. 2015, 35, 863–878. [Google Scholar] [CrossRef]
- Kusano, Y.; Madsen, B.; Berglund, L.; Aitomäki, Y.; Oksman, K. Dielectric barrier discharge plasma treatment of cellulose nanofibre surfaces. Surface Eng. 2017. [Google Scholar] [CrossRef]
- Weise, U.; Maloney, T.; Paulapuro, H. Quantification of water in different states of interaction with wood pulp fibres. Cellulose 1996, 3, 189–202. [Google Scholar] [CrossRef]
- Senthilnathan, J.; Weng, C.-C.; Liao, J.-D.; Yoshimura, M. Submerged liquid plasma for the synthesis of unconventional nitrogen polymers. Sci. Rep. 2013, 3, 2414. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, J.; Sanjeeva Rao, K.; Yoshimura, M. Submerged liquid plasma—Low energy synthesis of nitrogen-doped graphene for electrochemical applications. J. Mater. Chem. A 2014, 2, 3332–3337. [Google Scholar] [CrossRef]
- Anpilov, A.M.; Barkhudarov, E.M.; Bark, Y.B.; Zadiraka, Y.V.; Christofi, M.; Kozlov, Y.N.; Kossyi, I.A.; Kop’ev, V.A.; Silakov, V.P.; Taktakishvili, M.I.; Temchin, S.M. Electric discharge in water as a source of UV radiation, ozone and hydrogen peroxid. J. Phys. D Appl. Phys. 2011, 34, 993–999. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bazavan, M.; Ionita, E.R.; Dinescu, G. Characteristics of a long and stable filamentary argon plasma jet generated in ambient atmosphere. Plasma Sources Sci. Technol. 2015, 24, 025033. [Google Scholar] [CrossRef]
- Dinescu, G.; Ionita, E.R.; Luciu, I.; Grisolia, C. Flexible small size radiofrequency plasma torch for Tokamak wall cleaning. Fusion Eng. Des. 2007, 82, 2311–2317. [Google Scholar] [CrossRef]
- Tsai, T.C.; Staack, D. Low-temperature polymer deposition in ambient air using a floating-electrode dielectric barrier discharge jet. Plasma Process. Polym. 2011, 8, 523–534. [Google Scholar] [CrossRef]
- Ionita, E.R.; Ionita, M.D.; Stancu, E.C.; Teodorescu, M.; Dinescu, G. Small size plasma tools for material processing at atmospheric pressure. Appl. Surf. Sci. 2009, 225, 5448–5452. [Google Scholar] [CrossRef]
- Stancu, E.C.; Piroi, D.; Magureanu, M.; Dinescu, G. Decomposition of methylene blue by a cold atmospheric pressure plasma jet source. In Proceedings of the 20th International Symposium on Plasma Chemistry, Philadelphia, PA, USA, 24–29 July 2011; p. 375. [Google Scholar]
- Ionita, M.D.; Vizireanu, S.; Stoica, S.D.; Ionita, M.; Pandele, A.M.; Cucu, A.; Stamatin, I.; Nistor, L.C.; Dinescu, G. Functionalization of carbon nanowalls by plasma jet in liquid treatment. Eur. Phys. J. D 2016, 70, 31. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Emam, H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using acid hydrolysis. Int. J. Biol. Macromol. 2018, 107, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Fatah, I.Y.A.; Khalil, H.P.S.A.; Hossain, M.S.; Aziz, A.A.; Davoudpour, Y.; Dungani, R.; Bhat, A. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers 2014, 6, 2611–2624. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.C.I.M.; Abadi, A.G.; Katas, H. Purification, characterization and comparative studies of spray-dried bacterial cellulose microparticles. Carbohydr. Polym. 2014, 99, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I.A.; Dimmick, R.M.; Wilson, L.D.; Headley, J.V. Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr. Polym. 2016, 136, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kiziltas, E.E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.M.; Alam, M.M.; Daniels, S.M. Moisture removal from natural jute fibre by plasma drying process. Plasma Chem. Plasma Process. 2012, 32, 249–258. [Google Scholar] [CrossRef]
- Scalici, T.; Fiore, V.; Valenza, A. Effect of plasma treatment on the properties of Arundo Donax, L. leaf fibres and its bio-based epoxy composites: A preliminary study. Compos. Part B 2016, 94, 167–175. [Google Scholar] [CrossRef]
- Sadova, S.F.; Pankratova, E.V. Low-temperature plasma surface modification of textiles made from natural fibers and advanced technologies. High Energy Chem. 2009, 43, 234–240. [Google Scholar] [CrossRef]
- Lindh, J.; Ruan, C.; Strømme, M.; Mihranyan, A. Preparation of porous cellulose beads via introduction of diamine spacers. Langmuir 2016, 32, 5600–5607. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Horii, F.; Hirai, A. In situ crystallization of bacterial cellulose II. Influences of different polymeric additives on the formation of celluloses Iα and Iβ at the early stage of incubation. Cellulose 1996, 3, 229–242. [Google Scholar] [CrossRef]
- Lee, C.M.; Kubicki, J.D.; Fan, B.; Zhong, L.; Jarvis, M.C.; Kim, S.H. Hydrogen-bonding network and OH stretch vibration of cellulose: Comparison of computational modeling with polarized IR and SFG spectra. J. Phys. Chem. B 2015, 119, 15138–15149. [Google Scholar] [CrossRef] [PubMed]
- Turki, A.; El Oudiani, A.; Msahli, S.; Sakli, F. Investigation of OH bond energy for chemically treated alfa fibers. Carbohydr. Polym. 2018, 186, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, L.; Deng, H.; Li, J.; He, B.; Sun, R.; Ouyang, P. Structural changes of bamboo cellulose in formic acid. BioResources 2008, 3, 297–315. [Google Scholar]
- Gu, J.; Hu, C.; Zhong, R.; Tu, D.; Yun, H.; Zhang, W.; Leu, S.-Y. Isolation of cellulose nanocrystals from medium density fiberboards. Carbohydr. Polym. 2017, 167, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chatjigakis, A.K.; Pappas, C.; Proxenia, N.; Kalantzi, O.; Rodis, P.; Polissiou, M. FT-IR Spectroscopic Determination of the Degree of Esterification of Cell Wall Pectins from Stored Peaches and Correlation to Textural Changes. Carbohydr. Polym. 1998, 37, 395–408. [Google Scholar] [CrossRef]
- Chen, N.; Lin, Q.; Rao, J.; Zeng, Q.; Luo, X. Environmentally friendly soy-based bio-adhesive: Preparation, characterization, and its application to plywood. BioResources 2012, 7, 4273–4283. [Google Scholar]
- Nashy, E.H.A.; Osman, O.; Mahmoud, A.A.; Ibrahim, M. Molecular spectroscopic study for suggested mechanism of chrome tanned leather. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 2012, 88, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, T.; Cabane, E.; Chanana, M.; Hass, P.; Merk, V.; Gierlinger, N.; Burgert, I. A versatile strategy for grafting polymers to wood cell walls. Acta Biomater. 2015, 11, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Caschera, D.; Mezzi, A.; Cerri, L.; de Caro, T.; Riccucci, C.; Ingo, G.M.; Padeletti, G.; Biasiucci, M.; Gigli, G.; Cortese, B. Effects of plasma treatments for improving extreme wettability behavior of cotton fabrics. Cellulose 2014, 21, 741–756. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Giridhar, P. Fourier transform infrared spectroscopy (FTIR) analysis, chlorophyll content and antioxidant properties of native and defatted foliage of green leafy vegetables. J. Food Sci. Technol. 2015, 52, 8131–8139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, B.; Long, P.; He, B.; Yi, S.; Ou, B.; Shen, S.; Chen, J. Reversible fluorescence modulation of spiropyran functionalized carbon nanoparticles. J. Mater. Chem. C 2013, 1, 3716–3721. [Google Scholar] [CrossRef]
- Jansen, R.J.J.; van Bekkum, H. XPS of nitrogen-containing functional groups on activated carbon. Carbon 1995, 33, 1021–1027. [Google Scholar] [CrossRef]
- Qaiser, A.A.; Hyland, M.M. X-ray photoelectron spectroscopy characterization of polyaniline-cellulose ester composite membranes. Mater. Sci. Forum 2010, 657, 35–45. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-Ray Photoelectron Spectroscopic Characterization of Chemically Modified Electrodes Used as Chemical Sensors and Biosensors: A Review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Song, J.; Lv, D.; Wang, D.; Jaye, C.; Fischer, D.A.; Wu, T.; Chen, Y. Mechanism of enhanced carbon cathode performance by nitrogen doping in lithium−sulfur battery: An X-ray absorption spectroscopic study. J. Phys. Chem. C 2014, 118, 7765–7771. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, S.; Yang, M.; Chen, Z.; Ran, S. Thermo-Mechanical Performance of Polylactide Composites Reinforced with Alkali-Treated Bamboo Fibers. Polymers 2018, 10, 401. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, N.; Li, X.; Xu, S. Effect of inorganic–organic surface modification of calcium sulfate whiskers on mechanical and thermal properties of calcium sulfate whisker/poly(vinyl chloride) composites. RSC Adv. 2017, 7, 46486–46498. [Google Scholar] [CrossRef] [Green Version]









| Samples | Ar flow (sccm) | Reactive Gas/Liquid | RF Power (W) | Treatment Time (min) |
|---|---|---|---|---|
| NC Ar | 3000 | - | 100 | 30 |
| NC Ar/O2 | 3000 | O2 (5 sccm) | 100 | 30 |
| NC Ar/N2 | 3000 | N2 (10 sccm) | 100 | 30 |
| NC Ar/N2 (E15) | 2000 | N2 (1500 sccm) | 250 | 15 |
| NC Ar/N2 (E30) | 2000 | N2 (1500 sccm) | 250 | 30 |
| NC Ar/NH3 | 3000 | NH3 (5 sccm) | 100 | 30 |
| NC Ar-ACN | 3000 | ACN 30% in water | 100 | 30 |
| Samples | T10% (°C) | Ton (°C) | Tmax (°C) | RI (%) | T’max (°C) | R (%) |
|---|---|---|---|---|---|---|
| NC | 244.0 | 284.1 | 332.1 | - | - | 10.6 |
| NC Ar | 278.6 | 310.2 | 350.3 | - | - | 5.5 |
| NC Ar/O2 | 228.5 | 261.0 | 309.0 | 22.5 | 436.9 | 7.6 |
| NC Ar/N2 | 261.4 | 294.3 | 350.1 | - | - | 8.1 |
| NC Ar/N2 (E15) | 250.4 | 290.2 | 343.6 | 23.8 | 474.5 | 4.1 |
| NC Ar/N2 (E30) | 241.9 | 282.6 | 340.2 | 31.8 | 486.4 | 6.5 |
| NC Ar/NH3 | 237.3 | 268.4 | 323.6 | 20.4 | 436.6 | 6.9 |
| NC Ar-ACN | 241.2 | 289.0 | 333.8 | - | - | 8.3 |
| Samples | C1s (%) | O1s (%) | N1s (%) | O/C | N/C |
|---|---|---|---|---|---|
| NC | 63.8 | 35.0 | 1.2 | 0.55 | 0.02 |
| NC Ar | 62.9 | 36.2 | 0.9 | 0.57 | 0.01 |
| NC Ar/O2 | 63.5 | 35.1 | 1.4 | 0.55 | 0.02 |
| NC Ar/N2 | 64.6 | 33.9 | 1.5 | 0.52 | 0.02 |
| NC Ar/N2 (E15) | 61.4 | 37.5 | 1.1 | 0.61 | 0.02 |
| NC Ar/N2 (E30) | 61.0 | 38.2 | 0.8 | 0.63 | 0.01 |
| NC Ar/NH3 | 65.4 | 33.2 | 1.4 | 0.51 | 0.02 |
| NC Ar-ACN | 63.2 | 34.4 | 2.4 | 0.54 | 0.04 |
| Samples | C1s Components | O1s Components | |||||
|---|---|---|---|---|---|---|---|
| C1 (%) | C2 (%) | C3 (%) | C4 (%) | O1 (%) | O2 (%) | O3 (%) | |
| NC | 19.7 | 55.3 | 20.7 | 4.2 | 2.9 | 87.5 | 9.6 |
| NC Ar | 17.9 | 53.2 | 23.5 | 5.4 | 3.1 | 85.2 | 11.7 |
| NC Ar/O2 | 20.2 | 56.8 | 20.1 | 2.9 | 3.1 | 88.9 | 8.1 |
| NC Ar/N2 | 23.1 | 49.4 | 23.6 | 4.0 | 4.0 | 85.0 | 11.0 |
| NC Ar/N2 (E15) | 12.5 | 66.9 | 19.7 | 0.8 | 2.2 | 96.1 | 1.7 |
| NC Ar/N2 (E30) | 12.1 | 66.9 | 20.3 | 0.7 | 1.9 | 96.4 | 1.8 |
| NC Ar/NH3 | 24.9 | 54.5 | 17.6 | 2.9 | 3.6 | 88.0 | 8.4 |
| NC Ar-ACN | 18.3 | 53.6 | 22.6 | 5.6 | 3.4 | 82.7 | 13.9 |
| Nanocomposites | Tensile Strength (MPa) | Young’s Modulus (MPa) | Ton (°C) | Tmax (°C) |
|---|---|---|---|---|
| PHB-NC | 26.0 ± 1.4 | 1324 ± 62 | 269.0 | 281.9 |
| PHB-NC Ar | 29.5 ± 0.7 | 1358 ± 49 | 262.2 | 276.0 |
| PHB-NC Ar/O2 | 29.0 ± 1.1 | 1387 ± 59 | 263.6 | 277.2 |
| PHB-NC Ar/N2 | 28.5 ± 0.6 | 1395 ± 30 | 260.2 | 273.8 |
| PHB-NC Ar/N2 (E15) | 29.1 ± 0.5 | 1307 ± 41 | 265.5 | 279.5 |
| PHB-NC Ar/N2 (E30) | 29.8 ± 0.5 | 1309 ± 55 | 268.9 | 282.5 |
| PHB-NC Ar/NH3 | 29.2 ± 0.9 | 1402 ± 55 | 260.8 | 273.0 |
| PHB-NC Ar-ACN | 30.6 ± 0.3 | 1408 ± 22 | 269.1 | 281.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaitescu, D.M.; Vizireanu, S.; Nicolae, C.A.; Frone, A.N.; Casarica, A.; Carpen, L.G.; Dinescu, G. Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization. Nanomaterials 2018, 8, 467. https://doi.org/10.3390/nano8070467
Panaitescu DM, Vizireanu S, Nicolae CA, Frone AN, Casarica A, Carpen LG, Dinescu G. Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization. Nanomaterials. 2018; 8(7):467. https://doi.org/10.3390/nano8070467
Chicago/Turabian StylePanaitescu, Denis Mihaela, Sorin Vizireanu, Cristian Andi Nicolae, Adriana Nicoleta Frone, Angela Casarica, Lavinia Gabriela Carpen, and Gheorghe Dinescu. 2018. "Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization" Nanomaterials 8, no. 7: 467. https://doi.org/10.3390/nano8070467





