Figure 1.
Schematic diagram of flow-between and flow-through electrodes in CDI cells.
Figure 1.
Schematic diagram of flow-between and flow-through electrodes in CDI cells.
Figure 2.
Schematic of a flow-through cell (a) and cross-section of flow-distributor chamber (b).
Figure 2.
Schematic of a flow-through cell (a) and cross-section of flow-distributor chamber (b).
Figure 3.
(a) Conductivity variations of NaCl solution in the adsorption and release time for 3.52 wt% a-SWCNT-coated RVC electrodes at a flow-rate of 25 mL/min with various applied voltages and operating times; and (b) electrosorption as a function of applied voltage.
Figure 3.
(a) Conductivity variations of NaCl solution in the adsorption and release time for 3.52 wt% a-SWCNT-coated RVC electrodes at a flow-rate of 25 mL/min with various applied voltages and operating times; and (b) electrosorption as a function of applied voltage.
Figure 4.
Variations in the conductivity of in the adsorption and release of NaCl solution at different applied flow rates and operating times when measured at 1.5 V: (a) 3.63 wt%; (b) 12.50 wt%; (c) 17.43 wt%; and (d) 23.58 wt% a-SWCNT-coated RVC electrodes.
Figure 4.
Variations in the conductivity of in the adsorption and release of NaCl solution at different applied flow rates and operating times when measured at 1.5 V: (a) 3.63 wt%; (b) 12.50 wt%; (c) 17.43 wt%; and (d) 23.58 wt% a-SWCNT-coated RVC electrodes.
Figure 5.
(a) Adsorption and release behaviour of various a-SWCNT-coated RVC electrodes at 1.5 volt, using 50 mL/min water flow-rate between electrode; and (b) the electrosorption capacity in terms of mass of a-SWCNTs and in terms of the geometric volume of the composite electrode at various loadings of a-SWCNTs on the RVC electrode using the CDI system.
Figure 5.
(a) Adsorption and release behaviour of various a-SWCNT-coated RVC electrodes at 1.5 volt, using 50 mL/min water flow-rate between electrode; and (b) the electrosorption capacity in terms of mass of a-SWCNTs and in terms of the geometric volume of the composite electrode at various loadings of a-SWCNTs on the RVC electrode using the CDI system.
Figure 6.
Comparison of NaCl adsorption and the release behaviour of the 23.58 wt% a-SWCNT-coated RVC electrode when configured for flow-between and flow-through systems in a CDI cell measured at a flow rate of 50 mL/min, 75 ppm NaCl, and 1.5 V.
Figure 6.
Comparison of NaCl adsorption and the release behaviour of the 23.58 wt% a-SWCNT-coated RVC electrode when configured for flow-between and flow-through systems in a CDI cell measured at a flow rate of 50 mL/min, 75 ppm NaCl, and 1.5 V.
Figure 7.
Conductivity variations of the NaCl solution for the 23.58 wt% a-SWCNT-coated RVC electrode as a function of operation time with respect to various flow rates (25 mL/min, 50 mL/min, and 75 mL/min) measured at 1.5 V.
Figure 7.
Conductivity variations of the NaCl solution for the 23.58 wt% a-SWCNT-coated RVC electrode as a function of operation time with respect to various flow rates (25 mL/min, 50 mL/min, and 75 mL/min) measured at 1.5 V.
Figure 8.
The effect of distance between the electrodes (5 mm, 15 mm, and 25 mm) on the current and conductivity behaviour of the 23.58 wt% a-SWCNT-coated RVC electrode measured at a voltage of 1.5 V and a flow rate of 50 mL/min.
Figure 8.
The effect of distance between the electrodes (5 mm, 15 mm, and 25 mm) on the current and conductivity behaviour of the 23.58 wt% a-SWCNT-coated RVC electrode measured at a voltage of 1.5 V and a flow rate of 50 mL/min.
Figure 9.
(a) Adsorption and release behaviour; and (b) the electrosorption capacity in terms of the mass of a-SWCNT and the geometric volume of the electrode at various a-SWCNT-coated RVC electrode coatings of a-SWCNT (wt%): 3.63%, 12.50%, 17.43%, and 23.58%.
Figure 9.
(a) Adsorption and release behaviour; and (b) the electrosorption capacity in terms of the mass of a-SWCNT and the geometric volume of the electrode at various a-SWCNT-coated RVC electrode coatings of a-SWCNT (wt%): 3.63%, 12.50%, 17.43%, and 23.58%.
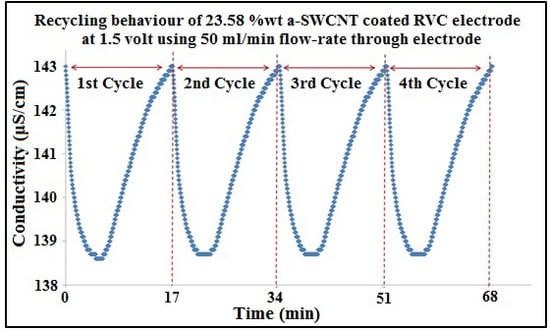
Figure 10.
Multiple electrosorption–desorption cycles of 75 ppm NaCl solution for the 23.58 wt% a-SWCNT-coated RVC electrode. Polarization and depolarization were performed at 1.5 V and 0.0 V, respectively.
Figure 10.
Multiple electrosorption–desorption cycles of 75 ppm NaCl solution for the 23.58 wt% a-SWCNT-coated RVC electrode. Polarization and depolarization were performed at 1.5 V and 0.0 V, respectively.
Figure 11.
The (a) electrosorption; (b) intraparticle diffusion; (c) pseudo-first-order adsorption kinetics; and (d) pseudo-second-order adsorption kinetics of NaCl onto the 23.58 wt% a-SWCNT-coated RVC electrode at a flow rate of 50 mL/min and a voltage of 1.5 V.
Figure 11.
The (a) electrosorption; (b) intraparticle diffusion; (c) pseudo-first-order adsorption kinetics; and (d) pseudo-second-order adsorption kinetics of NaCl onto the 23.58 wt% a-SWCNT-coated RVC electrode at a flow rate of 50 mL/min and a voltage of 1.5 V.
Figure 12.
The electrosorption isotherm for the 23.58 wt% a-SWCNT-coated RVC electrode at a flow rate of 50 mL/min NaCl and a voltage of 1.5 V.
Figure 12.
The electrosorption isotherm for the 23.58 wt% a-SWCNT-coated RVC electrode at a flow rate of 50 mL/min NaCl and a voltage of 1.5 V.
Table 1.
Electrosorption capacity of various a-SWCNT-coated RVC electrodes.
Table 1.
Electrosorption capacity of various a-SWCNT-coated RVC electrodes.
| Sample | a-SWCNT in Sample (wt%) | Electrosorption Capacity |
|---|
| mg/g of a-SWCNT | mg/cm3 of Electrode | mg/cm2 of Electrode |
|---|
| 1 | 3.63 | 8.39 | 0.02 | 2.8 × 10−3 |
| 2 | 12.50 | 3.69 | 0.03 | 4.8 × 10−3 |
| 3 | 17.43 | 3.42 | 0.05 | 6.5 × 10−3 |
| 4 | 23.74 | 2.77 | 0.06 | 7.6 × 10−3 |
Table 2.
The current of the CDI system for different inter-electrode distances using 1.5 V.
Table 2.
The current of the CDI system for different inter-electrode distances using 1.5 V.
| Distance between Electrodes (mm) | Time Required for Adsorption (min) | Initial Current (mA) | Stable Current (mA) | Charge (C) | Energy (J) |
|---|
| 5 | 6 | 4.56 | 1.02 | 0.45 | 0.67 |
| 15 | 25 | 2.20 | 0.27 | 0.61 | 0.91 |
| 25 | 60 | 1.11 | 0.16 | 0.95 | 1.42 |
Table 3.
Electrosorption capacity of different a-SWCNT/RVC composite electrodes.
Table 3.
Electrosorption capacity of different a-SWCNT/RVC composite electrodes.
| Sample | a-SWCNT in Sample (wt%) | Electrosorption Capacity |
|---|
| mg/g of a-SWCNT | mg/cm3 of Electrode | mg/cm2 of Electrode |
|---|
| 1 | 3.63 | 10.40 | 0.03 | 3.5 × 10−3 |
| 2 | 12.50 | 3.99 | 0.05 | 5.1 × 10−3 |
| 3 | 17.43 | 3.67 | 0.06 | 7.2 × 10−3 |
| 4 | 23.58 | 3.23 | 0.08 | 9.4 × 10−3 |
Table 4.
Electrosorption capacities and the time of one desalination cycle of various a-SWCNT-coated RVC electrodes obtained by comparing the performance between flow feed-between (FB) electrodes and flow feed-through (FT) electrodes with the NaCl feed solution (75 mg/L).
Table 4.
Electrosorption capacities and the time of one desalination cycle of various a-SWCNT-coated RVC electrodes obtained by comparing the performance between flow feed-between (FB) electrodes and flow feed-through (FT) electrodes with the NaCl feed solution (75 mg/L).
| a-SWCNT in the RVC Electrode (wt%) | Flow Direction | Electrosorption (mg/g of a-SWCNT) | # Enhancement in Electrosorption (%) | Time of One Desalination Cycle (min) |
|---|
| 3.63 | FT | 10.40 | 23.96 | 10 |
| FB | 8.39 | - | 30 |
| 12.50 | FT | 3.99 | 8.13 | 12 |
| FB | 3.69 | - | 36 |
| 17.43 | FT | 3.67 | 7.31 | 15 |
| FB | 3.42 | - | 37 |
| 23.58 | FT | 3.23 | 16.61 | 18 |
| FB | 2.77 | - | 39 |
Table 5.
Different parameter values obtained using the pseudo-first-order model, pseudo-second-order model, and intraparticle diffusion.
Table 5.
Different parameter values obtained using the pseudo-first-order model, pseudo-second-order model, and intraparticle diffusion.
| Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion |
|---|
| qe (mg/g) | K1 (min−1) | R2 | qe (mg/g) | K2 (g/mg min) | R2 | h (mg/g min) | Kid (mg/g min0.5) | R2 |
|---|
| 3.19 | 0.82 | 0.994 | 4.72 | 0.123 | 0.950 | 2.74 | 1.66 | 0.981 |
Table 6.
Determined parameters of regression coefficients R2, KL, and KF of Langmuir and Freundlich isotherms for NaCl electrosorption by using the a-SWCNT (23.58 wt%)-coated RVC electrode as an electrosorption electrode.
Table 6.
Determined parameters of regression coefficients R2, KL, and KF of Langmuir and Freundlich isotherms for NaCl electrosorption by using the a-SWCNT (23.58 wt%)-coated RVC electrode as an electrosorption electrode.
| Isotherm | Parameter | Value |
|---|
| Langmuir | qm | 13.08 |
| KL | 0.01 |
| R2 | 0.997 |
| Freundlich | KF | 0.28 |
| n | 1.77 |
| R2 | 0.989 |
Table 7.
Comparison of electrosorption capacity of various carbon electrodes and electrodes developed in this article.
Table 7.
Comparison of electrosorption capacity of various carbon electrodes and electrodes developed in this article.
| Parameters | Carbon Electrodes | Electrodes Developed in This Article (SWCNT/RVC) Using a New Cell |
|---|
| AC | CA | CNT | CNT/CNF | rGO | ACF | 3.63 wt% | 12.50 wt% | 17.43 wt% | 23.58 wt% |
|---|
| Electrosorption capacity (mg/g) | 1.51 | 2.56 | 2.33 | 3.32 | 3.23 | 4.64 | 10.40 | 3.99 | 3.67 | 3.23 |
| Electrosorption Capacity (mg/cm3) | - | - | - | - | - | - | 0.029 | 0.043 | 0.058 | 0.075 |
| Electrosorption capacity (mg/cm2) | - | - | - | - | - | - | 0.039 | 0.057 | 0.078 | 0.10 |
| Mass of materials (g) | 1.50 | 4.30 | 0.12 | 0.85 | 1.50 | 0.31 | 0.006 | 0.023 | 0.034 | 0.050 |
| Electrode length and width (cm) | 14 × 7 | 16 × 8 | 8 × 8 | 8.7 Diameter | 14 × 7 | 7 × 5 | 4 × 1.8 |
| Electrode thickness (mm) | 0.3 | 0.8 | 0.03 | 0.3 | 0.3 | 0.2 | 3.0 |
| Applied voltage (V) | 1.5 | 1.5 | 1.2 | 1.2 | 1.5 | 1.6 | 1.5 |
| Flow rate (mL/min) | 20 | 400 | 40 | 14 | 20 | 5 | 50 |
| Solution volume (mL) | 200 | 500 | 50 | 40 | 200 | 50 | 70 |
| Initial conductivity of NaCl solution (µS/cm) | 145 | 101 | 100 | 100 | 145 | 192 | 143 |
| Time of electrosorption cycle (min) | 30 | 840 | 100 | 45 | 30 | 150 | 6 |
| Reference | [24] | [25] | [22] | [23] | [24] | [21] | |