Highly Efficient and Reusable Montmorillonite/Fe3O4/Humic Acid Nanocomposites for Simultaneous Removal of Cr(VI) and Aniline
Abstract
:1. Introduction
2. Chemicals and Methods
2.1. Chemicals
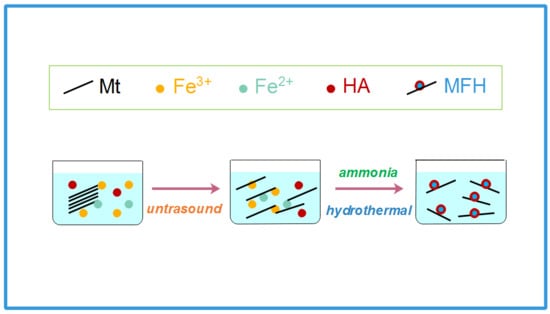
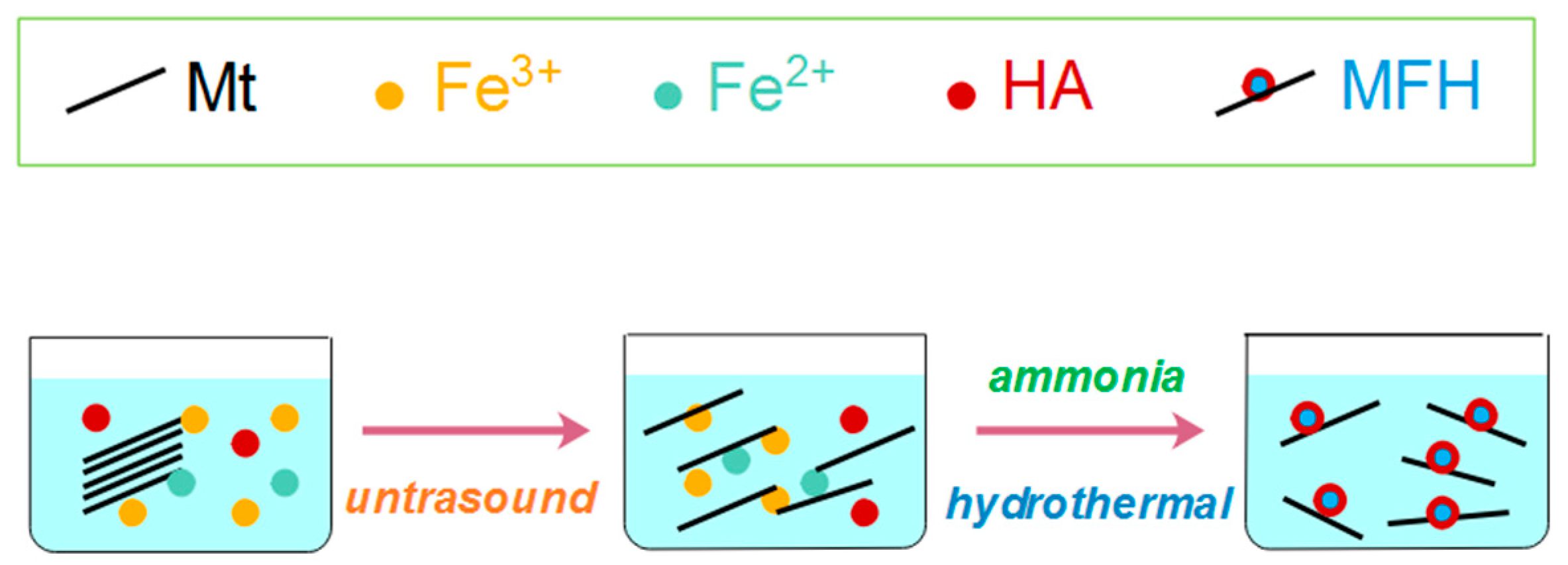
2.2. Fabrication of MFH
2.3. Characterization
2.4. Batch Adsorption Experiments
3. Results and Discussion
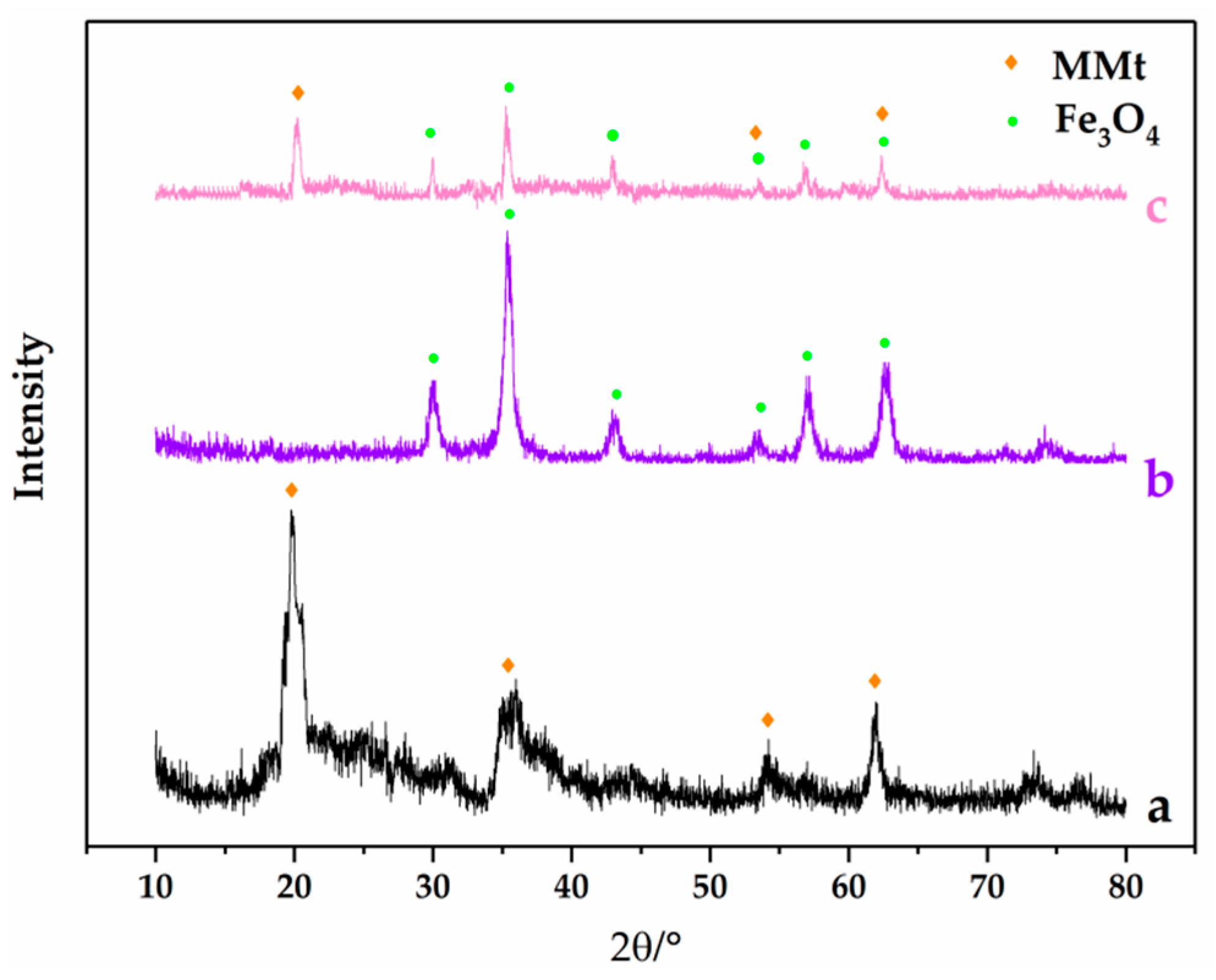
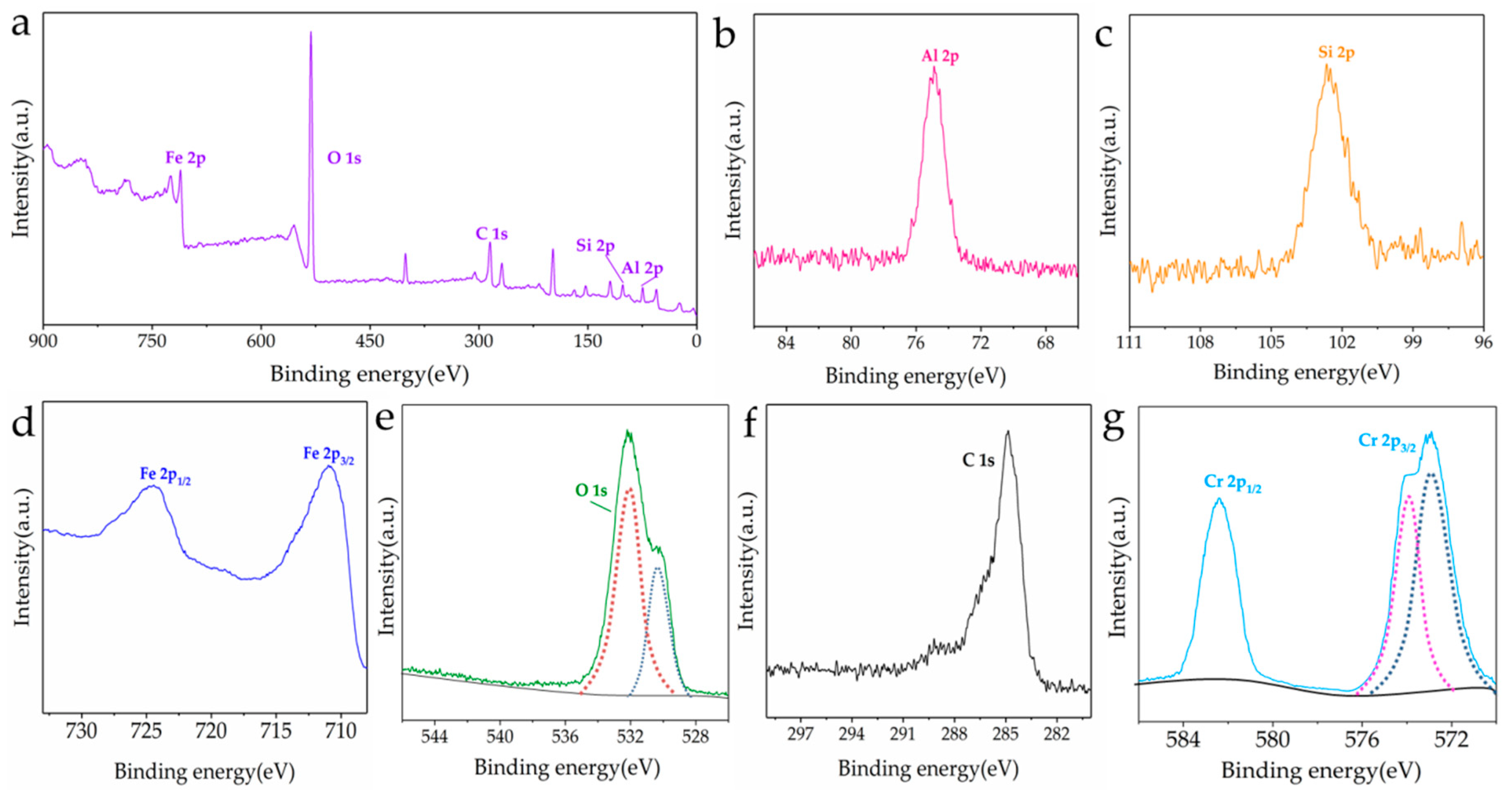
3.1. Characterization
3.2. Simultaneous Removal of Cr(VI) and Aniline
3.3. Adsorption Kinetics
3.4. Adsorption Isotherm
3.5. Effect of pH
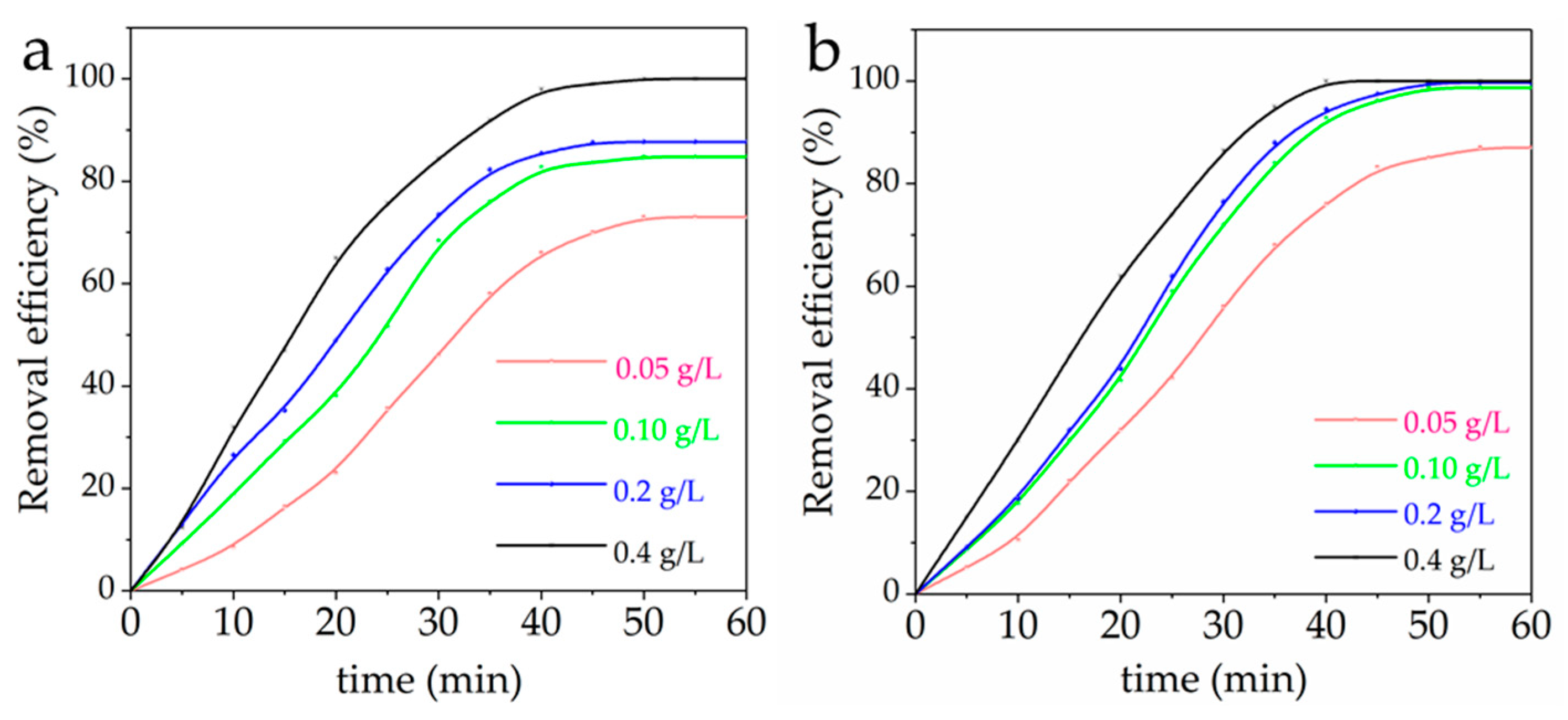
3.6. Effect of MFH Dosage
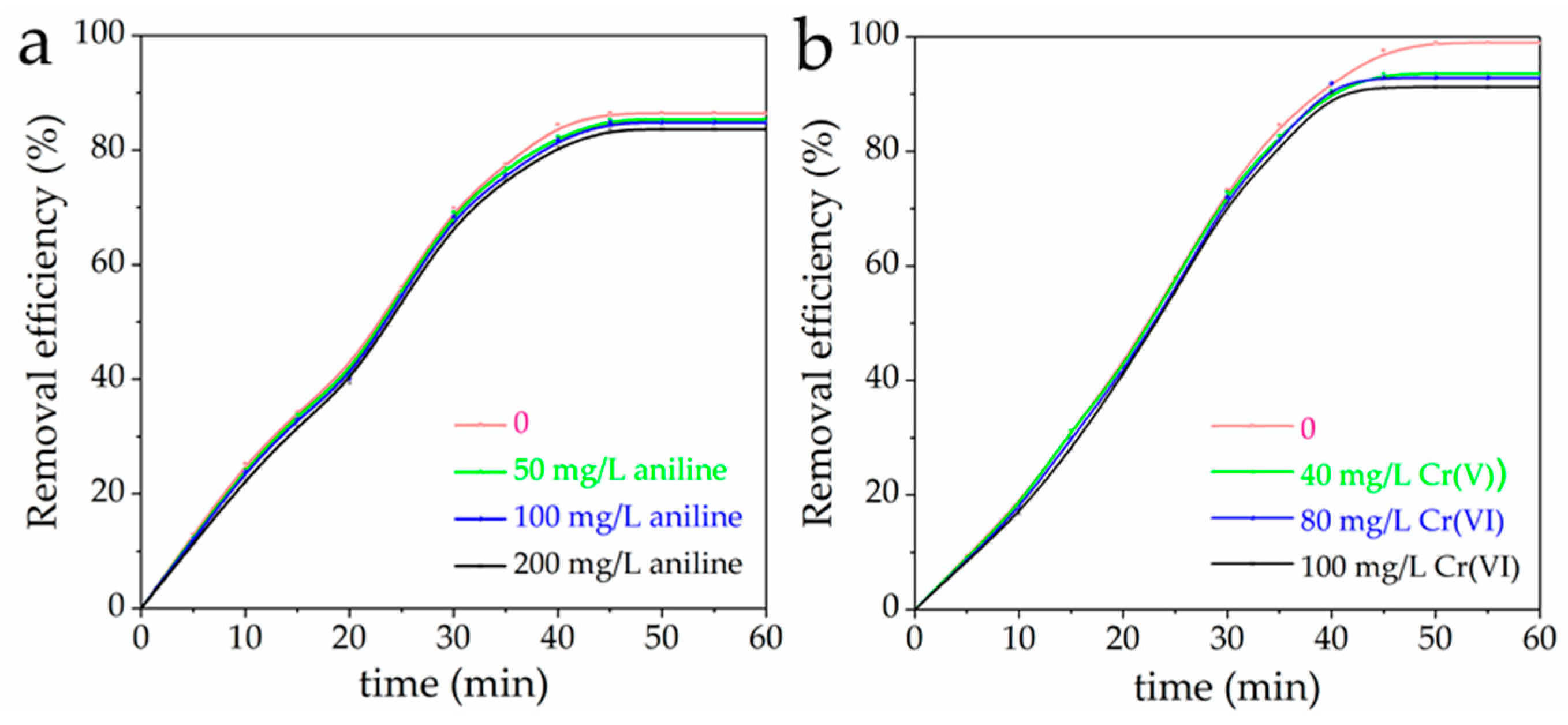
3.7. Mutual Effect between Cr(VI) and Aniline
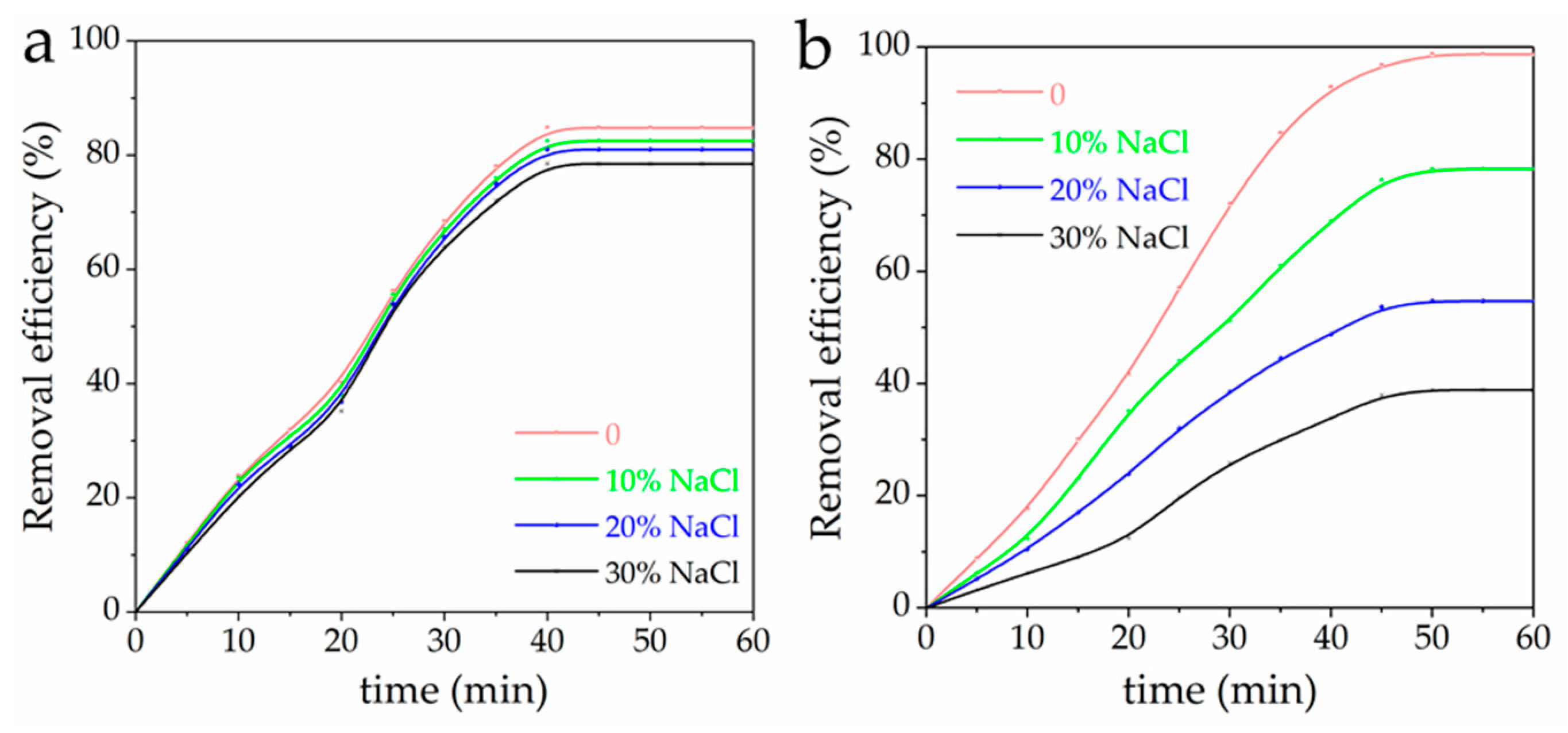
3.8. Effect of NaCl Content
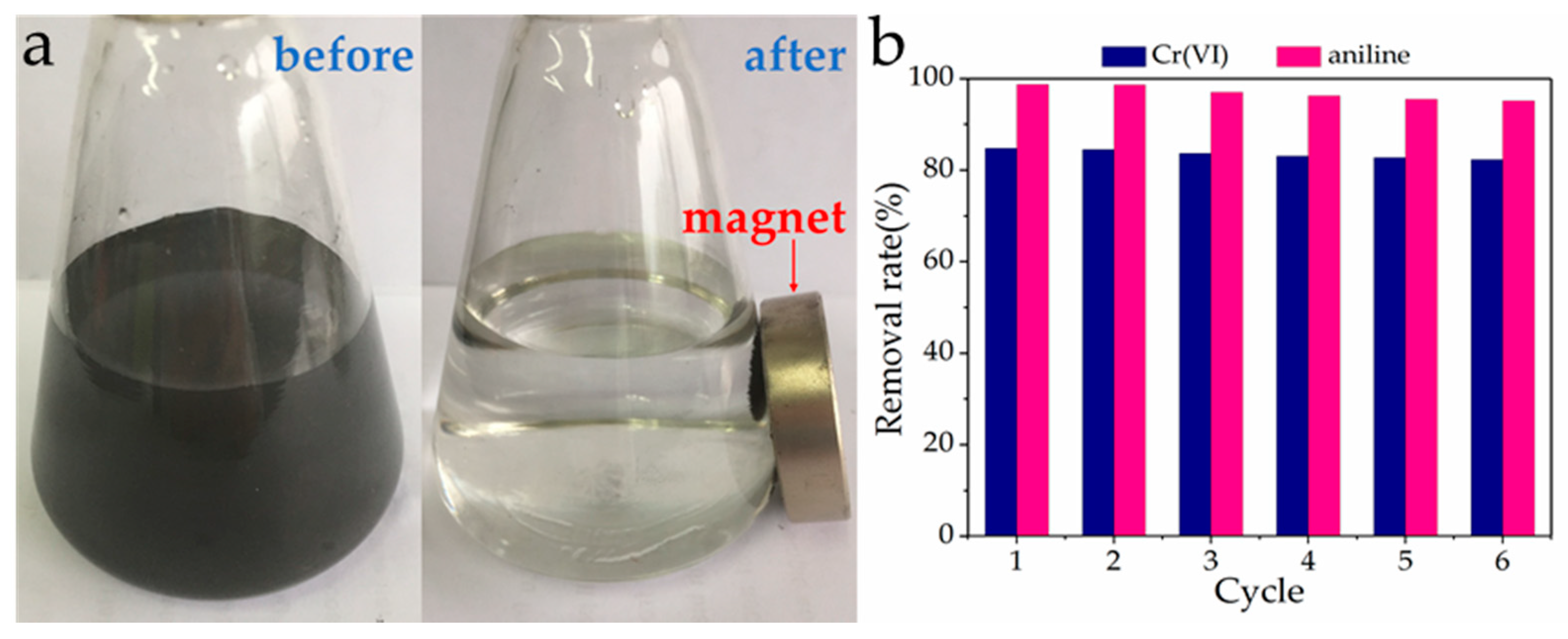
3.9. Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate. J. Hazard. Mater. 2018, 344, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Luo, H.; Yin, Y.; Zhou, W. Facile synthesis of MoS2/reduced graphene oxide composites for efficient removal of Cr(VI) from aqueous solutions. RSC Adv. 2017, 7, 24149–24156. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, J.K.; Hao, H.X.; Wang, T. Magnetically Separable MoS2/Fe3O4/nZVI Nanocomposites for the Treatment of Wastewater Containing Cr(VI) and 4-Chlorophenol. Nanomaterials 2017, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gao, M.L.; Luo, Z.X.; Ye, Y.G.; Liu, Y.N. Bis-pyridinium dibromides modified organo-bentonite for the removal of aniline from wastewater: A positive role of π–π polar interaction. Appl. Surf. Sci. 2014, 290, 107–115. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, L.; Xiao, S.; Chen, J.M. Preparation of a novel manganese oxide-modified diatomite and its aniline removal mechanism from solution. Chem. Eng. J. 2016, 284, 609–619. [Google Scholar] [CrossRef]
- Huang, R.; Zheng, D.; Yang, B.; Wang, B. Preparation and simultaneous sorption of CTMAB-HTCC bentonite towards aniline and Cr (VI). Energy Source Part A 2016, 38, 519–526. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Karakuzu, E.; Baybara, A.S.; Tuna, K.; Pehlivan, E. Utilization of eco-friendly gelatin for Cr(VI) adsorption. Desalin. Water Treat. 2017, 73, 308–315. [Google Scholar] [CrossRef]
- Chen, C.Y.; Geng, X.H.; Huang, W.L. Adsorption of 4-chlorophenol and aniline by nanosized activated carbons. Chem. Eng. J. 2017, 327, 941–952. [Google Scholar] [CrossRef]
- Ontañon, O.M.; González, P.S.; Barros, G.G.; Agostini, E. Improvement of simultaneous Cr (VI) and phenol removal by an immobilised bacterial consortium and characterisation of biodegradation products. New Biotechnol. 2017, 37, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Jin, X.D.; Zhao, N.N.; Angelidaki, I.; Zhang, Y.F. Efficient treatment of aniline containing wastewater in bipolar membrane microbial electrolysis cell-Fenton system. Water Res. 2017, 119, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhou, H.; Lv, Z.; Liu, H.; Guo, H. Sn4+ self-doped hollow cubic SnS as an efficient visible-light photocatalyst for Cr (VI) reduction and detoxification of cyanide. J. Mater. Chem. A 2017, 5, 6299–6309. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Shahmoradi, B.; Beikmohammadi, M.; Azizi, E.; Hossini, H.; Md, A.G. Photocatalytic degradation of Aniline from aqueous solutions under sunlight illumination using immobilized Cr: ZnO nanoparticles. Sci. Rep. 2017, 7, 1473. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Prior, M.T.; Gómez-Bombarelli, R.; González-Sánchez, M.I.; Valero, E. Biocatalytic oxidation of phenolic compounds by bovine methemoglobin in the presence of H2O2: Quantitative structure–activity relationships. J. Hazard. Mater. 2012, 241–242, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Pérez-Prior, M.T.; González-Sánchez, M.I. Removal of aromatic compounds from wastewater by hemoglobin soluble and immobilized on Eupergit® CM. Environ. Eng. Manag. J. 2014, 13, 2459–2466. [Google Scholar]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Zhou, L.; Wang, M.; Cai, D.; Wu, Z. Synthesis of a Multifunctional Graphene Oxide-Based Magnetic Nanocomposite for Efficient Removal of Cr(VI). Langmuir 2017, 33, 7007–7014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Arya, D.K.; Singh, N.; Vats, H.K. Removal of Cr (VI) Using Low Cost Activated Carbon Developed by Agricultural Waste. IOSR J. Appl. Chem. 2017, 10, 76–79. [Google Scholar] [CrossRef]
- Lv, G.; Li, Z.; Jiang, W.; Ackley, C.; Fenske, N.; Demarco, N. Removal of Cr(VI) from water using Fe(II)-modified natural zeolite. Chem. Eng. Res. Des. 2014, 92, 384–390. [Google Scholar] [CrossRef]
- Ma, L.; Xi, Y.; He, H.; Ayoko, G.A.; Zhu, R.; Zhu, J. Efficiency of Fe-montmorillonite on the removal of Rhodamine B and hexavalent chromium from aqueous solution. Appl. Clay Sci. 2016, 120, 9–15. [Google Scholar] [CrossRef]
- Lu, H.J.; Wang, J.K.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H.X. Mechanism, Synthesis and Modification of Nano Zerovalent Iron in Water Treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Ahmad, M.B.; Shameli, K.; Hussein, B.M.Z.; Khandanlou, R.; Khanehzaei, H. Size-Controlled synthesis of Fe3O4 magnetic nanoparticles in the layers of montmorillonite. J. Nanomater. 2014, 2014, 181. [Google Scholar] [CrossRef]
- Zhang, P.; Mo, Z.L.; Han, L.J.; Zhu, X.B.; Wang, B.; Zhang, C. Preparation and Photocatalytic Performance of Magnetic TiO2/Montmorillonite/Fe3O4 Nanocomposites. Ind. Eng. Chem. Res. 2014, 53, 8057–8061. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Hasanpour, S.; Behrouzi, L.; Sheykhloie, H. Study on adsorption of Cu(II) on magnetic starch-g-polyamidoxime/montmorillonite/Fe3O4 nanocomposites as novel chelating ligands. Starch Stärke 2016, 68, 188–199. [Google Scholar] [CrossRef]
- Cai, J.; Lei, M.; Zhang, Q.; He, J.R.; Chen, T.; Liu, S.; Fu, S.H.; Li, T.T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part. A Appl. Sci. Manuf. 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Ills, E.; Tombacz, E. The role of variable surface charge and surface complexation in the absorption of humic acid on magnetite. Colloids Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Ills, E.; Tombacz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Zong, P.F.; Ren, X.M.; Wang, Q.; Wang, X.K. Rapid and Highly Efficient Preconcentration of Eu(III) by Core−Shell Structured Fe3O4@Humic Acid Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 6891–6900. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lo, I.; Chen, G. Removal of Cr(VI) by magnetite. Water Sci. Technol. 2004, 50, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, G.X.; Jiang, X.L.; Xiao, Q.Q.; Niu, M.X.; Wang, Z.Y.; Wang, Y.Y. Non-biological reduction of Cr(VI) by reacting with humic acids composted from cattle manure. RSC Adv. 2017, 7, 26903–26911. [Google Scholar] [CrossRef]
- Jiang, W.J.; Cai, Q.; Xu, W.; Yang, M.W.; Cai, Y.; Dionysiou, D.D.; O’Shea, E.K. Cr(VI) Adsorption and Reduction by Humic Acid Coated on Magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Franson, M.A.H. Standard Method for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Belkova, A.A.; Sergeeva, A.I.; Apel, P.Y.; Beklemishev, M.K. Diffusion of aniline through a polyethylene terephthalate track-etched membrane. J. Membr. Sci. 2009, 330, 145–155. [Google Scholar] [CrossRef]
- Lian, G.; Zhang, X.; Zhang, S.J.; Liu, D.; Cui, D.L.; Wang, Q.L. Controlled fabrication of ultrathin-shell BN hollow spheres with excellent performance in hydrogen storage and wastewater treatment. Energy Environ. Sci. 2012, 5, 7072–7080. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.H.; Xu, L.Q.; Wang, L.C.; Ju, Z.C.; Li, G.D.; Qian, Y.T. High yield synthesis of novel boron nitride submicro-boxes and their photocatalytic application under visible light irradiation. Catal. Sci. Technol. 2011, 1, 1159–1165. [Google Scholar] [CrossRef]
- Masih, D.; Izumi, Y.; Aika, K.; Seida, Y. Optimization of an Iron Intercalated Montmorillonite Preparation for the Removalof Arsenic at Low Concentration. Eng. Life Sci. 2007, 7, 52–60. [Google Scholar] [CrossRef]
- Ebitani, K.; Ide, M.; Mitsudome, T.; Mizugaki, T.; Kaneda, K. Creation of a chain-like cationic iron species in montmorillonite as a highly active heterogeneous catalyst for alkane oxygenations using hydrogen peroxide. Chem. Commun. 2002, 7, 690–691. [Google Scholar] [CrossRef]
- Khang, V.C.; Korovkin, M.V.; Ananyeva, L.G. Identification of clay minerals in reservoir rocks by FTIR spectroscopy. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2016; Volume 43, p. 012004. [Google Scholar]
- Liu, Y.; Li, Y.; Zhao, X.; Chi, W.; Huang, Q.; Yu, C.; Xiang, Y. Effect of magnetite nanoparticles on dye absorption properties of magnetite@carbon composites. Bull. Mater. Sci. 2017, 40, 367–373. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High Efficient Removal of Heavy Metals in Water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Deng, S.; Wu, R.; Hong, S.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Highly efficient removal of hexavalent chromium from electroplating wastewater using aminated wheat straw. RSC Adv. 2016, 6, 8797–8805. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J.; Warchoł, J.K. Synthesis and Characterization of Two-Dimensional Transition Metal Dichalcogenide Magnetic MoS2@Fe3O4 Nanoparticles for Adsorption of Cr(VI)/Cr(III). ACS Omega 2017, 2, 6187–6200. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, D.H.; Zheng, Y.; Liang, S.P.; Liu, Z. Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater. 2009, 167, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, C.; Barios, J.I.; Caetano, M.; Farran, A.; Cortina, J.L. Kinetic evaluation of phenol/aniline mixtures adsorption from aqueous solutions onto activated carbon and hypercrosslinked polymeric resin (MN200). React. Funct. Polym. 2010, 70, 142–150. [Google Scholar] [CrossRef]
- Al-Johani, H.; Abdel, S.M. Kinetics and thermodynamic study of aniline adsorption by multi-walled carbon nanotubes from aqueous solution. J. Colloid Interface Sci. 2011, 360, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Stromer, B.S.; Woodbury, B.; Williams, C.F. Tylosin sorption to diatomaceous earth described by Langmuir isotherm and Freundlich isotherm models. Chemosphere 2018, 193, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Baseri, H.; Tizro, S. Treatment of nickel ions from contaminated water by magnetite based nanocomposite adsorbents: Effects of thermodynamic and kinetic parameters and modeling with Langmuir and Freundlich isotherms. Process Saf. Environ. 2017, 109, 465–477. [Google Scholar] [CrossRef]
- Fakhri, A. Adsorption characteristics of graphene oxide as a solid adsorbent for aniline removal from aqueous solutions: Kinetics, thermodynamics and mechanism studies. J. Saudi Chem. Soc. 2017, 21, S52–S57. [Google Scholar] [CrossRef]
- Li, L.; Duan, H.; Wang, X.; Luo, C. Adsorption property of Cr(VI) on magnetic mesoporous titanium dioxide–graphene oxide core–shell microspheres. New J. Chem. 2014, 38, 6008–6016. [Google Scholar] [CrossRef]
- Fan, L.L.; Luo, C.N.; Sun, M.; Qiu, H.M. Synthesis of graphene oxide decorated with magnetic cyclodextrin for fast chromium removal. J. Mater. Chem. 2012, 22, 24577–24583. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Enhanced removal of Cr (VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kakavandi, B.; Jafari, A.J.; Kalantary, R.R.; Nasseri, S.; Ameri, A.; Esrafily, A. Synthesis and properties of Fe3O4-activated carbon magnetic nanoparticles for removal of aniline from aqueous solution: Equilibrium, kinetic and thermodynamic studies. Iran. J. Environ. Health Sci. Eng. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- He, Y.T.; Traina, S.J. Cr(VI) reduction and immobilization by magnetite under alkaline pH conditions: The role of passivation. Environ. Sci. Technol. 2005, 39, 4499–4504. [Google Scholar] [CrossRef] [PubMed]
- Eary, L.; Rai, D. Chromate reduction by subsurface soils under acidic conditions. Soil Sci. Soc. Am. J. 1991, 55, 676–683. [Google Scholar] [CrossRef]
- Hashem, F.S. Removal of methylene blue by magnetite-covered bentonite nano-composite. Eur. Chem. Bull. 2013, 2, 524–529. [Google Scholar]





| Material | SBET (m2∙g−1) | Vtp (cm3∙g−1) | Dp (nm) | |
|---|---|---|---|---|
| Bare Fe3O4 NPs | 77.24 | 0.24 | 7.5 | |
| Mt powder | 53.72 | 0.12 | 8.6 | |
| MFH nanocomposites (Fe3O4:Mt, mass ratio) | 1:20 | 62.13 | 0.13 | 8.0 |
| 1:10 | 76.45 | 0.15 | 7.6 | |
| 1:4 | 98.86 | 0.19 | 6.5 | |
| 1:1 | 73.46 | 0.20 | 7.7 | |
| Model | Parameter | Cr(VI) | Aniline |
|---|---|---|---|
| qe,exp (mg∙g−1) | 339.25 ± 2.42 | 292.16 ± 2.06 | |
| Pseudo first-order | qe,cal (mg∙g−1) | 340.31 ± 2.97 | 293.83 ± 2.64 |
| K1 (min−1) | 0.132 ± 0.002 | 0.109 ± 0.001 | |
| R2 | 0.986 ± 0.001 | 0.911 ± 0.002 | |
| Pseudo second-order | qe,cal (mg∙g−1) | 341.19 ± 2.80 | 294.22 ± 2.31 |
| k2 (g∙mg−1∙min−1) | 0.0165 ± 0.0003 | 0.0274 ± 0.0005 | |
| R2 | 0.929 ± 0.001 | 0.982 ± 0.001 | |
| Intra particle diffusion | ki (mg∙g−1·min−1/2) | 8.24 ± 0.01 | 11.37 ± 0.02 |
| C | 0.103 ± 0.001 | 0.307 ± 0.002 | |
| R2 | 0.942 ± 0.001 | 0.975 ± 0.001 |
| Model | Parameter | Cr(VI) | Aniline |
|---|---|---|---|
| Langmuir | qm (mg∙g−1) | 374.19 ± 3.11 | 393.53 ± 3.32 |
| b (L∙mg−1) | 0.018 ± 0.001 | 0.096 ± 0.001 | |
| R2 | 0.995 ± 0.001 | 0.941 ± 0.001 | |
| Freundlich | K (mg1−(1/n) L1/n g−1) | 0.121 ± 0.001 | 16.58 ± 0.09 |
| n | 1.87 ± 0.01 | 3.74 ± 0.02 | |
| R2 | 0.907 ± 0.002 | 0.993 ± 0.001 |
| Adsorbate | Adsorbent | Adsorption Capacity (mg∙g−1) | Condition | Reference |
|---|---|---|---|---|
| Cr(VI) | Fe3O4@mTiO2@GO | 117.94 | pH = 2.0, T = 303 K | [49] |
| Graphene oxide decorated with magnetic cyclodextrin | 120 | pH = 3.0, T = 298 K | [50] | |
| PPY/Fe3O4 | 243.9 | pH = 2.0, T = 298 K | [51] | |
| MFH nanocomposites | 374.19 | pH = 3.0, T = 298 K | this study | |
| Aniline | Fe3O4-activated carbon | 90.91 | pH = 6.0, T = 293 K | [52] |
| Fe3O4/graphene | 202.84 | pH = 3.0, T = 298 K | [46] | |
| MFH nanocomposites | 393.53 | pH = 3.0, T = 298 K | this study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Wang, J.; Li, F.; Huang, X.; Tian, B.; Hao, H. Highly Efficient and Reusable Montmorillonite/Fe3O4/Humic Acid Nanocomposites for Simultaneous Removal of Cr(VI) and Aniline. Nanomaterials 2018, 8, 537. https://doi.org/10.3390/nano8070537
Lu H, Wang J, Li F, Huang X, Tian B, Hao H. Highly Efficient and Reusable Montmorillonite/Fe3O4/Humic Acid Nanocomposites for Simultaneous Removal of Cr(VI) and Aniline. Nanomaterials. 2018; 8(7):537. https://doi.org/10.3390/nano8070537
Chicago/Turabian StyleLu, Haijiao, Jingkang Wang, Fei Li, Xin Huang, Beiqian Tian, and Hongxun Hao. 2018. "Highly Efficient and Reusable Montmorillonite/Fe3O4/Humic Acid Nanocomposites for Simultaneous Removal of Cr(VI) and Aniline" Nanomaterials 8, no. 7: 537. https://doi.org/10.3390/nano8070537