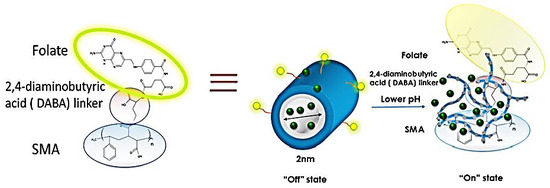
Functionalized Folic Acid-Conjugated Amphiphilic Alternating Copolymer Actively Targets 3D Multicellular Tumour Spheroids and Delivers the Hydrophobic Drug to the Inner Core
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Synthesis of Folic-DABA Ligands
2.3. Synthesis of Folic-DABA-Poly (Styrene-Alt-Maleic Anhydride) (PSMA) Nanoparticles
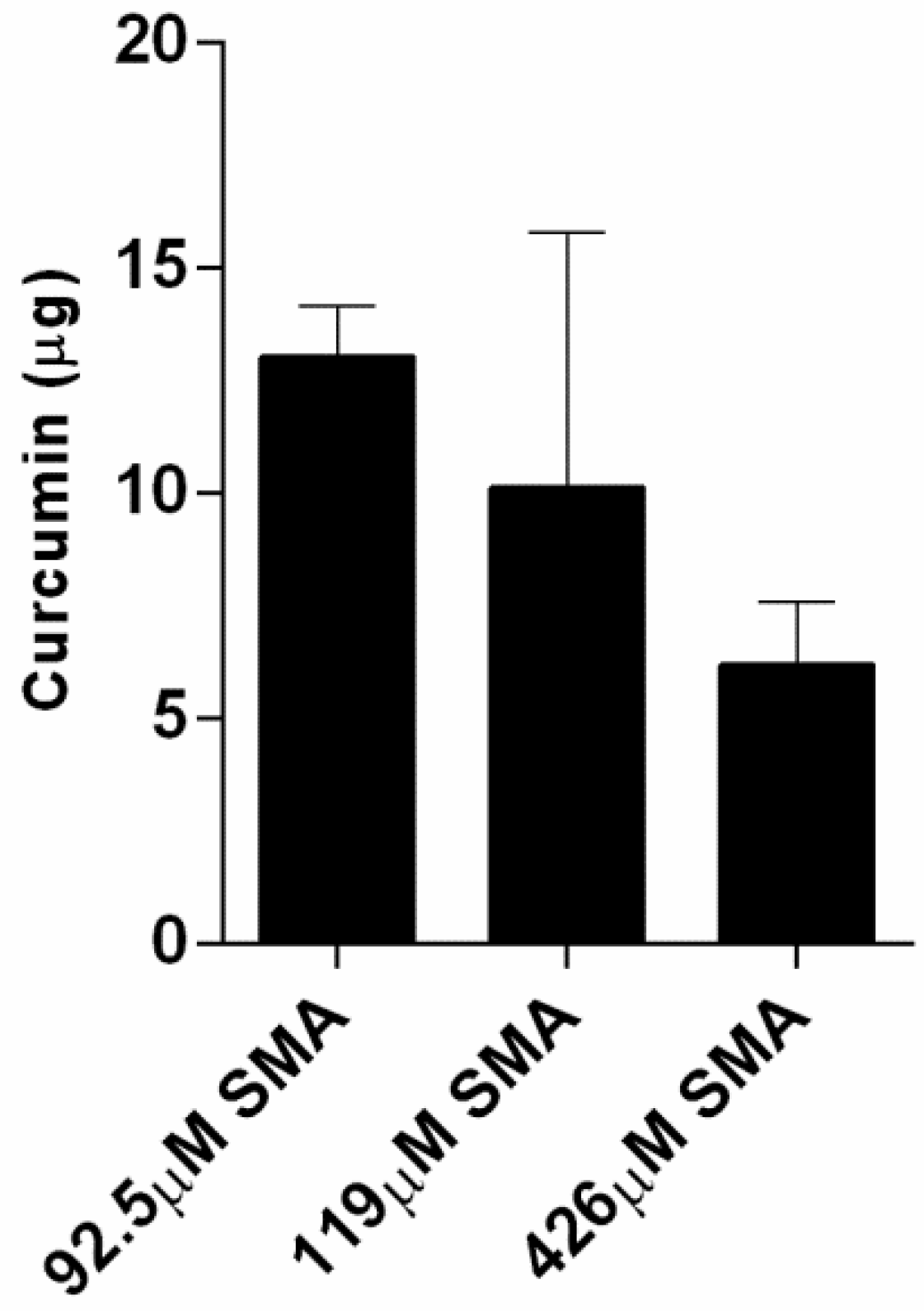
2.4. Curcumin Loading Capacity with SMA and FA-DABA-SMA Polymers
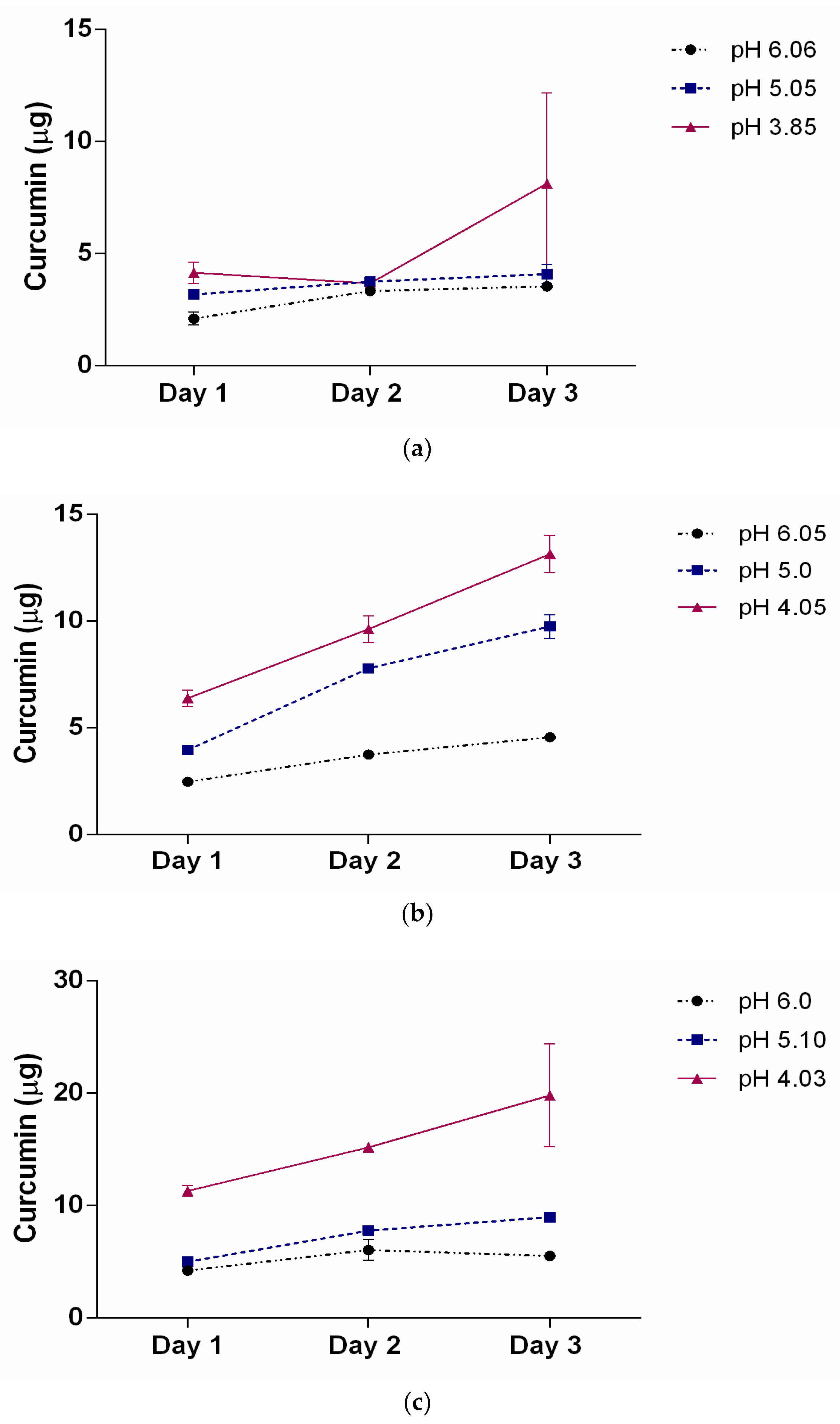
2.5. Curcumin-Loaded SMA Polymers Release Profile
2.6. WST-1 Cell Proliferation Assay
2.7. Generation of 3D Multicellular Tumour Spheroids
2.8. Spheroid Volume Measurements as a Measure of Treatment Efficacy
2.9. Fluorescent Microscopy of Curcumin-Loaded SMA and FA-DABA-SMA Polymers Targeting 3D Multicellular Tumour Spheroids
2.10. Statistical Software
3. Results and Discussion
3.1. Characterization of Hydrophobic Drugs Encapsulation by SMA and Release Profiles
3.2. SMA and FA-DABA-SMA Polymers Encapsulated with Curcumin Targeting Breast Tumour Spheroids
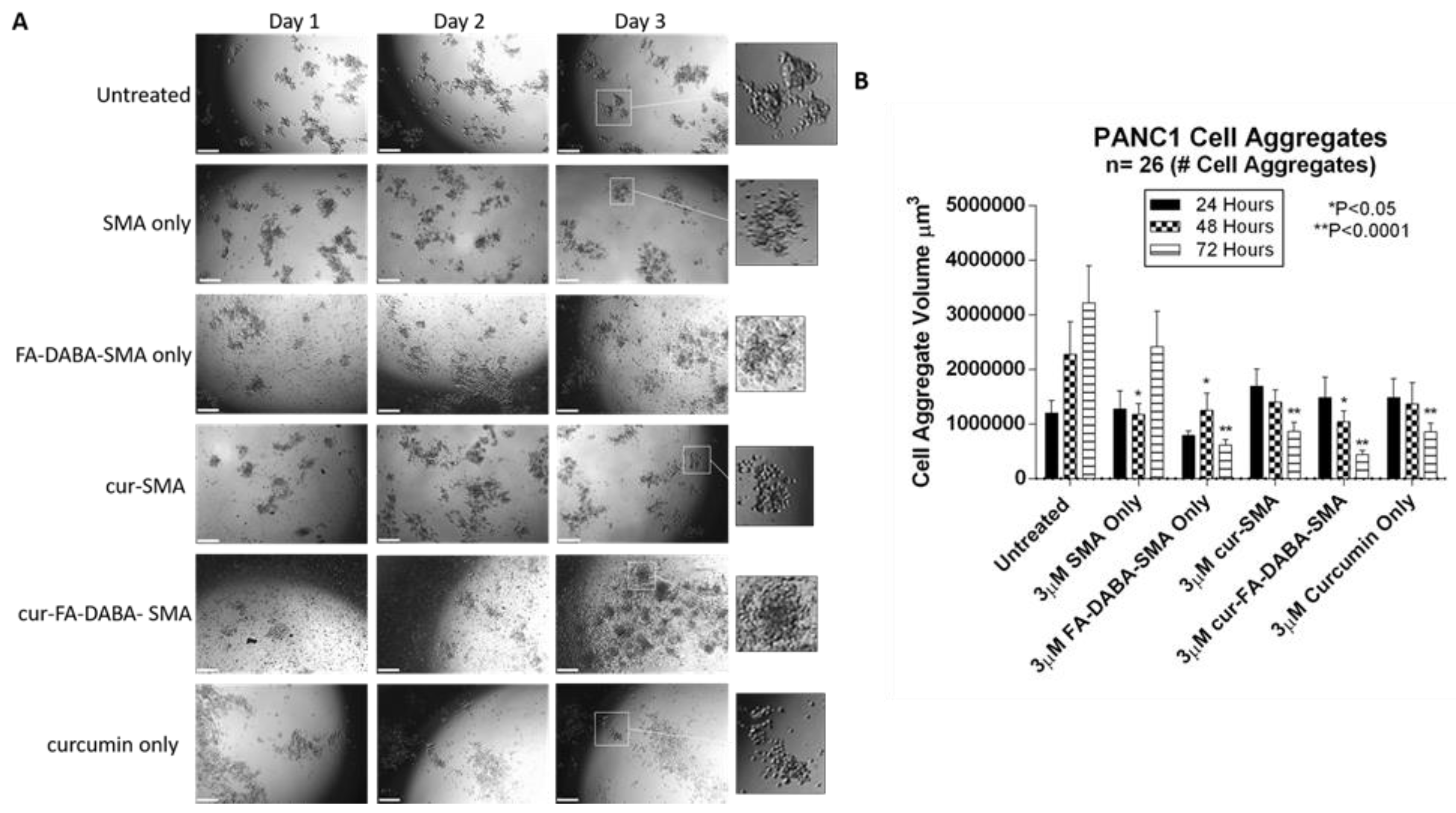
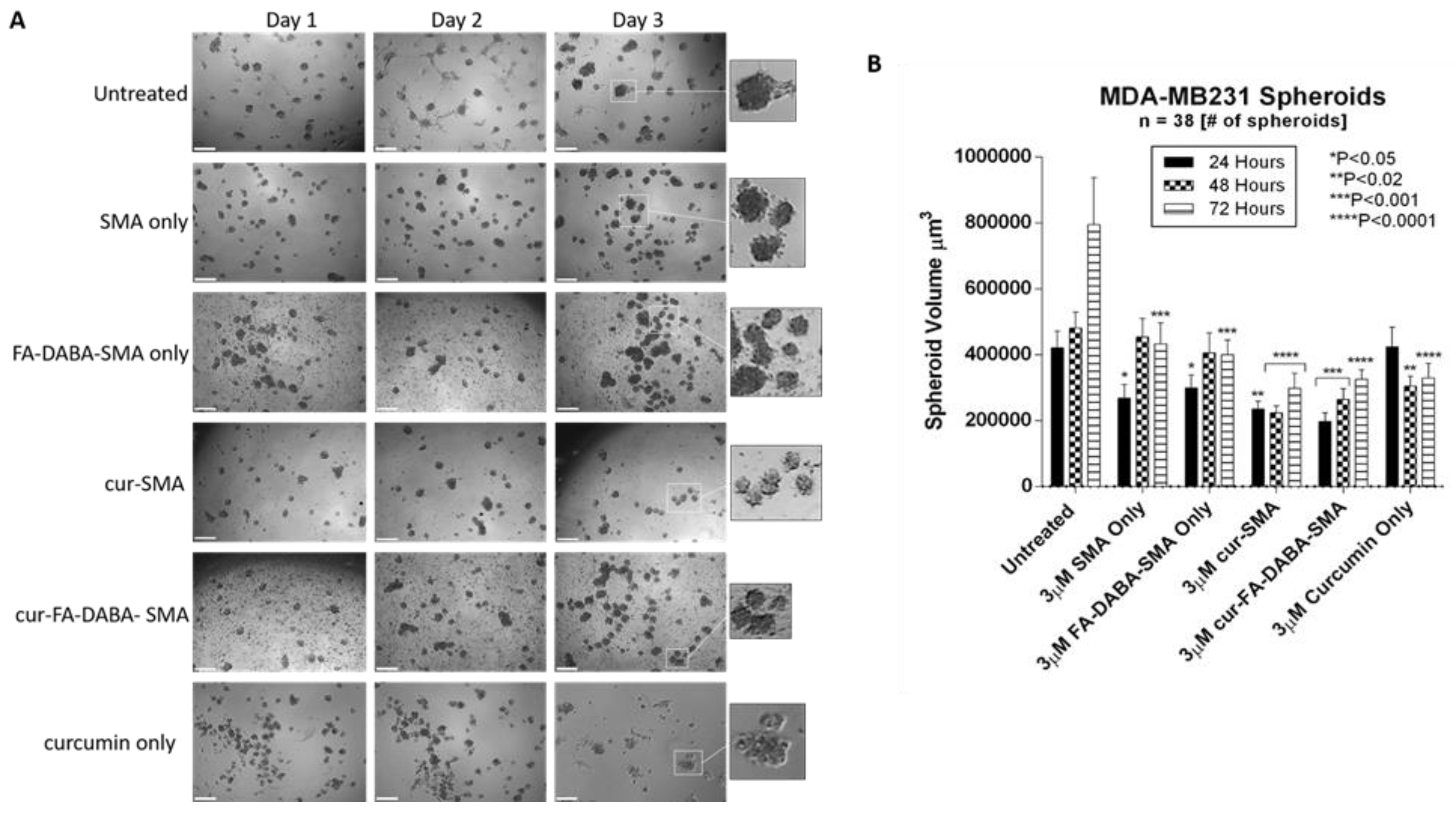
3.3. Changes in Tumour Spheroid Volume of PANC-1 and MDA-MB231 Cells Following Treatment with Empty and Curcumin Loaded SMA and FA-DABA-SMA Polymers
3.4. The Effects of Clinical Standard Hydrophobic Chemotherapeutics Drug Loaded Functionalized FA-DABA-SMA Polymers on Cell Viability Using Monolayer Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-FU | fluorouracil |
| Cur | curcumin |
| DABA | 2,4-diaminobutyric acid |
| ECM | extracellular matrix |
| EPR | enhanced permeability and retention |
| FA-SMA | folate functionalized SMA |
| FRα | folate acid receptor alpha |
| MCTS | multicellular tumour spheroid(s) |
| MDA-MB231 | human triple negative breast cancer cell line |
| NRP1 | neuropilin-1 |
| PANC-1 | human pancreatic cancer cell line |
| PEG | polyethylene glycol |
| pHe | extracellular pH |
| RGDfK | Arg-Gly-Asp-D-Phe-Lys |
| SMA | poly(styrene-alt-maleic anhydride) |
| TPP | triphenyl phosphonium cation |
| Wt | weight |
References
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.; Alzhrani, R.M.; Kesharwani, P.; Sau, S.; Boddu, S.H.; Iyer, A.K. Folate Decorated Nanomicelles Loaded with a Potent Curcumin Analogue for Targeting Retinoblastoma. Pharmaceutics 2017, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Zou, Z.; Xin, K.; Bian, X.; Cai, X.; Lu, W.; Chen, J.; Chen, G.; Huang, L.; Blair, A.M.; et al. Tumor-Penetrating Peptide Fused Egfr Single-Domain Antibody Enhances Cancer Drug Penetration into 3d Multicellular Spheroids and Facilitates Effective Gastric Cancer Therapy. J. Control. Release 2015, 200, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Priwitaningrum, D.L.; Blonde, J.G.; Sridhar, A.; van Baarlen, J.; Hennink, W.E.; Storm, G.; Le Gac, S.; Prakash, J. Tumor Stroma-Containing 3d Spheroid Arrays: A Tool to Study Nanoparticle Penetration. J. Control. Release 2016, 244, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Waite, C.L.; Roth, C.M. Pamam-Rgd Conjugates Enhance Sirna Delivery through a Multicellular Spheroid Model of Malignant Glioma. Bioconjugate Chem. 2009, 20, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science (New York) 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer Treatment by Targeted Drug Delivery to Tumor Vasculature in a Mouse Model. Science (New York) 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Murphy, E.A.; Majeti, B.K.; Barnes, L.A.; Makale, M.; Weis, S.M.; Lutu-Fuga, K.; Wrasidlo, W.; Cheresh, D.A. Nanoparticle-Mediated Drug Delivery to Tumor Vasculature Suppresses Metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 9343–9348. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.; Zaytseva-Zotova, D.; Burov, S.; Leko, M.; Dontenwill, M.; Chiper, M.; Vandamme, T.; Markvicheva, E. Formation of Multicellular Tumor Spheroids Induced by Cyclic Rgd-Peptides and Use for Anticancer Drug Testing in vitro. Int. J. Pharm. 2016, 506, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.; Haq, S.; Haxho, F.; Samuel, V.; Burov, S.V.; Markvicheva, E.; Neufeld, R.J.; Szewczuk, M.R. Sialylation Transmogrifies Human Breast and Pancreatic Cancer Cells into 3d Multicellular Tumor Spheroids Using Cyclic Rgd-Peptide Induced Self-Assembly. Oncotarget 2016, 7, 66119–66134. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Samuel, V.; Haxho, F.; Akasov, R.; Leko, M.; Burov, S.V.; Markvicheva, E.; Szewczuk, M.R. Sialylation Facilitates Self-Assembly of 3d Multicellular Prostaspheres by Using Cyclo-Rgdfk(tpp) Peptide. Onco Targets Ther. 2017, 10, 2427–2447. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; McTaggart, M.; Malardier-Jugroot, C. Synthesis and Characterization of a Ph Responsive Folic Acid Functionalized Polymeric Drug Delivery System. Biophys. Chem. 2016, 214, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Szewczuk, M.R.; Malardier-Jugroot, C. Folic Acid-Conjugated Amphiphilic Alternating Copolymer as a New Active Tumor Targeting Drug Delivery Platform. Drug Des. Dev. Ther. 2016, 10, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Elnakat, H.; Ratnam, M. Distribution, Functionality and Gene Regulation of Folate Receptor Isoforms: Implications in Targeted Therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Shmeeda, H.; Mak, L.; Tzemach, D.; Astrahan, P.; Tarshish, M.; Gabizon, A. Intracellular Uptake and Intracavitary Targeting of Folate-Conjugated Liposomes in a Mouse Lymphoma Model with Up-Regulated Folate Receptors. Mol. Cancer Ther. 2006, 5, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Low, P.S. Immunotherapy of Folate Receptor-Expressing Tumors: Review of Recent Advances and Future Prospects. J. Control. Release. 2003, 91, 17–29. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate Receptor Expression in Carcinomas and Normal Tissues Determined by a Quantitative Radioligand Binding Assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Malardier-Jugroot, C.; van de Ven, T.G.; Cosgrove, T.; Richardson, R.M.; Whitehead, M.A. Novel Self-Assembly of Amphiphilic Copolymers into Nanotubes: Characterization by Small-Angle Neutron Scattering. Langmuir 2005, 21, 10179–10187. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The Epr Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. Wst-1-Based Cell Cytotoxicity Assay as a Substitute for Mtt-Based Assay for Rapid Detection of Toxigenic Bacillus Species Using Cho Cell Line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.; Gileva, A.; Zaytseva-Zotova, D.; Burov, S.; Chevalot, I.; Guedon, E.; Markvicheva, E. 3d in vitro Co-Culture Models Based on Normal Cells and Tumor Spheroids Formed by Cyclic Rgd-Peptide Induced Cell Self-Assembly. Biotechnol. Lett. 2017, 39, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative Cellular Uptake, Localization and Cytotoxicity of Curcumin in Normal and Tumor Cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Peng, S.F.; Lee, C.Y.; Lu, C.C.; Tsai, S.C.; Shieh, T.M.; Wu, T.S.; Tu, M.G.; Chen, M.Y.; Yang, J.S. Curcumin-Loaded Nanoparticles Induce Apoptotic Cell Death through Regulation of the Function of Mdr1 and Reactive Oxygen Species in Cisplatin-Resistant Car Human Oral Cancer Cells. Int. J. Oncol. 2013, 43, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Lee, J.; Huh, T.-L.; Lee, Y.S. Curcumin Induces Apoptosis in Human Colorectal Carcinoma (hct-15) Cells by Regulating Expression of Prp4 and p53. Mol. Cells 2013, 35, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sahoo, S.K. Folate Decorated Dual Drug Loaded Nanoparticle: Role of Curcumin in Enhancing Therapeutic Potential of Nutlin-3a by Reversing Nultidrug Resistance. PLoS ONE 2012, 7, e32920. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric Nanoparticle-Encapsulated Curcumin (“Nanocurcumin”): A Novel Strategy for Human Cancer Therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, X.; Pi, W.; Liu, S. Folic acid Inhibits Nasopharyngeal Cancer Cell Proliferation and Invasion via Activation of Fralpha/erk1/2/tslc1 Pathway. Biosci. Rep. 2017, 37, BSR20170772. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Chang, C.; Lee, W.S. Folic Acid Inhibits Colo-205 Colon Cancer Cell Proliferation through Activating the Fralpha/c-src/erk1/2/nfkappab/tp53 Pathway: In vitro and in vivo Studies. Sci. Rep. 2015, 5, 11187. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Novel Strategies to Improve the Anticancer Action of 5-Fluorouracil by Using Drug Delivery Systems. Molecules 2008, 13, 2340–2369. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Wang, X.; Gu, Y.; Wang, Y.; Wang, Y.; Gong, C.; Luo, F.; Guo, G.; Zhao, X.; Wei, Y.; et al. Self-Assembled Biodegradable Micelles Based on Star-Shaped Pcl-b-peg Copolymers for Chemotherapeutic Drug Delivery. Coll. Surf. A Physicochem. Eng. Aspects 2010, 358, 128–134. [Google Scholar] [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Luo, S.; Liu, T.; Jiang, Y.; Liu, S. Thiol and pH Dual-Responsive Dynamic Covalent Shell Cross-Linked Micelles for Triggered Release of Chemotherapeutic Drugs. Polym. Chem. 2013, 4, 695–706. [Google Scholar] [CrossRef]
- Boshnjaku, V.; Shim, K.W.; Tsurubuchi, T.; Ichi, S.; Szany, E.V.; Xi, G.; Mania-Farnell, B.; McLone, D.G.; Tomita, T.; Mayanil, C.S. Nuclear Localization of Folate Receptor Alpha: A New Role as a Transcription Factor. Sci. Rep. 2012, 2, 980. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.; Chelvam, V.; Low, P.S. Comparison of Nanoparticle Penetration into Solid Tumors and Sites of Inflammation: Studies Using Targeted and Nontargeted Liposomes. Nanomedicine 2015, 10, 1439–1449. [Google Scholar] [CrossRef] [PubMed]






| Name | Chemical Structure | Molecular Weight |
|---|---|---|
| Curcumin | 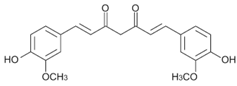 | 368.4 g·mol−1 |
| 5-Fluorouracil (5-FU) |  | 130.1 g·mol−1 |
| Paclitaxel | 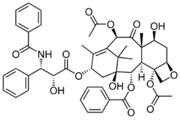 | 853.9 g·mol−1 |
| SMA Polymer Concentration | Curcumin Precipitation | Encapsulation Efficiency |
|---|---|---|
| 92.5 μM | 13 μg | 98.7% |
| 119.0 μM | 11 μg | 98.9% |
| 426.0 μM | 6 μg | 99.4% |
| Concentration of SMA | pH 4 | pH 5 | pH 6 | |||
|---|---|---|---|---|---|---|
| Mass | % Released | Mass | % Released | Mass | % Released | |
| 31.7 μM | 6.14 μg | 7.0% | 3.68 μg | 5.5% | 3.47 μg | 5.27% |
| 63.4 μM | 13.33 μg | 20.1% | 10.22 μg | 15.4% | 5.11 μg | 7.7% |
| 111 μM | 17.53 μg | 26.6% | 8.79 μg | 13.3% | 5.30 μg | 8% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sambi, M.; DeCarlo, A.; Burov, S.V.; Akasov, R.; Markvicheva, E.; Malardier-Jugroot, C.; Szewczuk, M.R. Functionalized Folic Acid-Conjugated Amphiphilic Alternating Copolymer Actively Targets 3D Multicellular Tumour Spheroids and Delivers the Hydrophobic Drug to the Inner Core. Nanomaterials 2018, 8, 588. https://doi.org/10.3390/nano8080588
Li X, Sambi M, DeCarlo A, Burov SV, Akasov R, Markvicheva E, Malardier-Jugroot C, Szewczuk MR. Functionalized Folic Acid-Conjugated Amphiphilic Alternating Copolymer Actively Targets 3D Multicellular Tumour Spheroids and Delivers the Hydrophobic Drug to the Inner Core. Nanomaterials. 2018; 8(8):588. https://doi.org/10.3390/nano8080588
Chicago/Turabian StyleLi, Xia, Manpreet Sambi, Alexandria DeCarlo, Sergey V. Burov, Roman Akasov, Elena Markvicheva, Cecile Malardier-Jugroot, and Myron R. Szewczuk. 2018. "Functionalized Folic Acid-Conjugated Amphiphilic Alternating Copolymer Actively Targets 3D Multicellular Tumour Spheroids and Delivers the Hydrophobic Drug to the Inner Core" Nanomaterials 8, no. 8: 588. https://doi.org/10.3390/nano8080588
APA StyleLi, X., Sambi, M., DeCarlo, A., Burov, S. V., Akasov, R., Markvicheva, E., Malardier-Jugroot, C., & Szewczuk, M. R. (2018). Functionalized Folic Acid-Conjugated Amphiphilic Alternating Copolymer Actively Targets 3D Multicellular Tumour Spheroids and Delivers the Hydrophobic Drug to the Inner Core. Nanomaterials, 8(8), 588. https://doi.org/10.3390/nano8080588