Effect of Temperature on the Physical, Electro-Chemical and Adsorption Properties of Carbon Micro-Spheres Using Hydrothermal Carbonization Process
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Temperature on Physical, Electrochemical, and Thermal Properties of Hydro-Char
2.1.1. Yield, Energy Content, pH, Electrical Conductivity, and Ultimate Analysis
2.1.2. Thermogravimetric/Proximate Analysis
2.1.3. Surface Morphology Analysis
2.1.4. Surface Area and Porosity Analysis
2.1.5. Crystallinity or X-ray Diffraction (XRD) Analysis
2.2. Equilibrium Adsorption Isotherm and Thermodynamic Parameters Evaluation
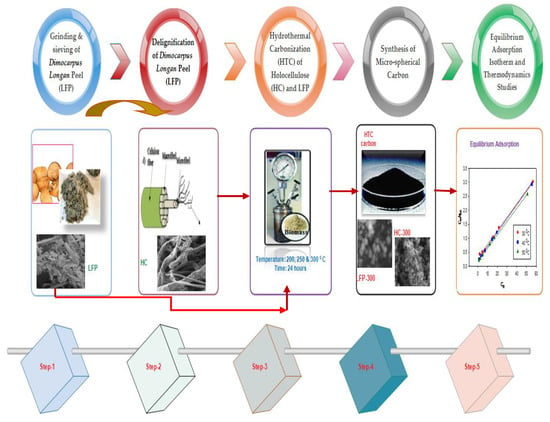
3. Materials and Methods
3.1. Grinding and Sieving of Dimocarpus longan Peel (LFP)
3.2. Delignification of Dimocarpus longan Peel (LFP)
3.3. Hydrothermal Carbonization of HC and LFP
3.4. Synthesis of Carbon Micro-Spheres and Physical and Electro-Chemical Characterizations
3.5. Equilibrium Adsorption Isotherm and Thermodynamics Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizhikovs, J.; Zandersons, J.; Spince, B.; Dobele, G.; Jakab, E. Preparation of granular activated carbon from hydrothermally treated and pelletized deciduous wood. J. Anal. Appl. Pyrol. 2011, 93, 68–76. [Google Scholar] [CrossRef]
- Robbins, M.P.; Evans, G.; Valentine, J.; Donnison, I.; Allison, G. New opportunities for the exploitation of energy crops by thermochemical conversion in Northern Europe and the UK. Prog. Energy Combust. Sci. 2012, 38, 138–155. [Google Scholar] [CrossRef]
- Luque, R.; Sudipta, D.; Alina, M.B. Catalytic Conversion of Biomass. Catalysts 2016, 6, 148. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Yu, S.H.; Müller, J.; Antonietti, M. A direct synthesis of mesoporous carbons with bi-continuous pore morphology from crude plant material by hydrothermal carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A.; Rahman, M.M.; Rafique, R.F. Catalytic activation and application of micro-spherical carbon derived from hydrothermal carbonization of lignocellulosic biomass: Statistical analysis using Box-Behnken design. RSC Adv. 2016, 6, 102680–102694. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Wang, J.; Shi, T.; Yang, H.; Wang, X. Characterization of products from hydrothermal liquefaction and carbonation of biomass model compounds and real biomass. J. Fuel Technol. 2011, 39, 893–900. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Garlof, S.; Mecklenburg, M.; Smazna, D.; Mishra, Y.K.; Adelung, R.P.; Schulte, S.; Fiedler, B. 3D carbon networks and their polymer composites: Fabrication and electromechanical investigations of neat Aerographite and Aerographite-based PNCs under compressive load. Carbon 2017, 111, 103–112. [Google Scholar] [CrossRef]
- Schütt, F.; Signetti, S.; Krüger, H.; Röder, S.; Smazna, D.; Kaps, S.; Gorb, S.N.; Mishra, Y.K.; Pugno, N.M.; Adelung, R. Hierarchical self-entangled carbon nanotube tube networks. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fan, L.Z.; Zhou, M.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Makowski, P.; Cakan, R.D.; Antonietti, M.; Goettmann, F.; Titirici, M. Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon. Chem. Commun. 2008, 8, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, S. Conducting performance of individual Ag@C coaxial nanocables: Ideal building blocks for interconnects in nanoscale devices. Small 2009, 5, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A.; Das, R.; Hasan, M.R.; Zain, S.M.; Khalid, K.; Uddin, M.N. Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 2013, 8, 6523–6555. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Karim, M.Z.; Ashraf, M.A.; Khalid, K. Influence of carbonization temperature on physicochemical properties of biochar derived from slow pyrolysis of durian wood (Durio zibethinus) sawdust. BioResources 2016, 11, 3356–3372. [Google Scholar] [CrossRef]
- Manzoli, M.; Menegazzo, F.; Signoretto, M.; Marchese, D. Biomass Derived Chemicals: Furfural Oxidative Esterification to Methyl-2-furoate over Gold Catalysts. Catalysts 2016, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Falco, C.; Sieben, J.M.; Brun, N.; Sevilla, M.; van der Mauelen, T.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M. Hydrothermal carbons from hemicellulose-derived aqueous hydrolysis products as electrode materials for supercapacitors. ChemSusChem 2013, 6, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Seehra, M.S.; Akkineni, L.P.; Yalamanchi, M.; Singh, V.; Poston, J. Structural characteristics of nanoparticles produced by hydrothermal pretreatment of cellulose and their applications for electrochemical hydrogen generation. Int. J. Hydrog. Energy 2012, 37, 9514–9523. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Wang, B.S.; Lin, W.C.; Huang, S.S.; Lee, C.Y.; Yen, M.T.; Huang, M.H. Antioxidant and Anti-Inflammatory Properties of Longan (Dimocarpus longan Lour.) Pericarp. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Herrero, M.; Hormaza, J.I. Fruiting pattern in Longan, Dimocarpus longan: From pollination to aril development. Ann. Appl. Biol. 2016, 169, 357–368. [Google Scholar] [CrossRef]
- Kumar, S.; Kothari, U.; Kong, L.; Lee, Y.Y.; Gupta, R.B. Hydrothermal pretreatment of switchgrass and corn stover for production of ethanol and carbon microspheres. Biomass Bioenergy 2011, 35, 956–968. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Wu, J.Q.; Yuan, T.Q.; Sun, R.C. Preparation and characterization of lignocellulosic oil sorbent by hydrothermal treatment of Populus fiber. Materials 2014, 7, 6733–6747. [Google Scholar] [CrossRef] [PubMed]
- Saqib, N.U.; Oh, M.; Jo, W.; Park, S.; Lee, J. Conversion of dry leaves into hydrochar through hydrothermal carbonization (HTC). J. Mater. Cycles Waste Manag. 2015, 19, 111–117. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Liu, F.; Guo, M. Comparison of the characteristics of hydrothermal carbons derived from holocellulose and crude biomass. J. Mater. Sci. 2015, 50, 1624–1631. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Ravindran, H.; Gupta, R.B.; Fasina, O.; Tu, M.; Fernando, S.D. Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour. Technol. 2010, 101, 8389–8395. [Google Scholar] [CrossRef] [PubMed]
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical modification of biomass residues during hydrothermal carbonization What makes the difference, temperature or feedstock? Org. Geochem. 2013, 54, 91–100. [Google Scholar] [CrossRef]
- Baçaoui, A.; Yaacoubi, A.; Dahbi, A.; Bennouna, C.; Luu, R.P.T.; Maldonado-Hodar, F.J.; Rivera-Utrilla, J.; Moreno-Castilla, C. Optimization of conditions for the preparation of activated carbons from olive-waste cakes. Carbon 2001, 39, 425–432. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T. Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J. Colloid Interface Sci. 2004, 274, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Danso-Boateng, E.; Holdich, R.G.; Shama, G.; Wheatley, A.D.; Sohail, M.; Martin, S.J. Kinetics of faecal biomass hydrothermal carbonisation for hydrochar production. Appl. Energy 2013, 111, 351–357. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.C.A.; Coscione, A.R.; Abreu, C.A.; Puga, A.P.; Camargo, O.A. Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw-derived biochar. BioResources 2013, 8, 4992–5004. [Google Scholar] [CrossRef]
- Grønli, M.G.; Várhegyi, G.; Di Blasi, C. Thermogravimetric analysis and devolatilization kinetics of wood. Ind. Eng. Chem. Res. 2002, 41, 4201–4208. [Google Scholar] [CrossRef]
- Uzun, B.B.; Pütün, A.E.; Pütün, E. Composition of products obtained via fast pyrolysis of olive-oil residue: Effect of pyrolysis temperature. J. Anal. Appl. Pyrol. 2007, 79, 147–153. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Adebisi, A.G.; Chowdhury, Z.Z.; Abd Hamid, S.B.; Ali, M.E. Activated carbons for removal of Pb(II) and Zn(II) prepared by phosphoric acid activation of hydrothermally treated banana empty fruit bunch using box-Behnken design. BioResources 2016, 11, 9686–9709. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. R. 2015, 22, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 4, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Recent modification to Langmuir isotherms. Acta Physicochem. 1940, 12, 217–225. [Google Scholar]
- Abd Hamid, S.B.; Chowdhury, Z.Z.; Zain, S.M. Base Catalytic Approach: A Promising Technique for Activation of Bio Char for Equilibrium Sorption Studies of Copper, Cu (II) ions in Single Solute System. Materials 2014, 7, 2815–2832. [Google Scholar] [CrossRef] [PubMed]
- Abideen, I.A.; Ofudje, A.E.; Mopelola, A.I.; Sarafadeen, O.K. Equilibrium kinetics and thermodynamics studies of the biosorption of Mn (II) ions from aqueous solution by raw and acid treated corn cob biomass. Am. J. Appl. Sci. 2011, 6, 302–309. [Google Scholar]
- Farheen, K.; Rizwan, W.; Mohamed, H.; Rua, A.L.; Mohd, R. Nanotransition Materials (NTMs): Photocatalysis, Validated High Effective Sorbent Models Study for Organic Dye Degradation and Precise Mathematical Data’s at Standardized Level. Nanomaterials 2018, 8, 134. [Google Scholar] [CrossRef]
- Adebisi, G.A.; Chowdhury, Z.Z.; Abd Hamid, S.B.; Ali, E. Equilibrium Isotherm, Kinetic, and Thermodynamic Studies of Divalent Cation Adsorption onto Calamus gracilis Sawdust-Based Activated Carbon. BioResources 2017, 12, 2872–2898. [Google Scholar] [CrossRef]
- Adebisi, G.A.; Chowdhury, Z.Z.; Alaba, P.A. Equilibrium, Kinetic, and Thermodynamic Studies of Lead ion and Zinc ion Adsorption from Aqueous Solution onto Activated Carbon Prepared from Palm Oil Mill Effluents. J. Clean. Prod. 2017, 148, 958–968. [Google Scholar] [CrossRef]
- Duran-Valle, C.J.; Gomez-Corzo, M.; Pastor-Villegas, J.; Gomez-Serrano, V. Study of Cherry Stones as raw materials in preparation of carbonaceous adsorbents. J. Anal. Appl. Pyrol. 2005, 73, 59–67. [Google Scholar] [CrossRef]
- Tongpoothorn, W.; Sriuttha, M.; Homchan, P.; Chanthai, S.; Ruangviriyachai, C. Preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation andcharacterization of their physico-chemical properties. Chem. Eng. Res. Des. 2011, 89, 335–340. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid, and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Abd Hameed, S.B.; Chowdhury, Z.Z.; Karim, M.Z. Catalytic Extraction of Microcrystalline Cellulose (MCC) from Elaeis guineensis using Central Composite Design (CCD). BioResources 2014, 9, 7403–7426. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Abd Hameed, S.B. Preparation and Characterization of Nano-crystalline Cellulose Using Ultrasonication Combined with a Microwave-assisted Pretreatment Process. BioResources 2016, 11, 397–3415. [Google Scholar] [CrossRef]








| Sample | Ultimate or CHNOS Analysis (wt %) | Yield | HHV | pH | EC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | H/C | O/C | % | MJ-Kg−1 | - | mS | |
| Raw LFP | 32.65 | 10.87 | 2.12 | 54.34 | 0.02 | 0.33 | 1.66 | - | 18.05 | - | - |
| LFP-200 | 40.78 | 7.66 | 2.02 | 49.51 | 0.03 | 0.19 | 1.21 | 56.45 | 18.95 | 4.92 | 0.97 |
| LFP-250 | 52.21 | 4.67 | 1.23 | 41.87 | 0.02 | 0.09 | 0.80 | 43.89 | 19.09 | 5.79 | 1.48 |
| LFP-300 | 70.88 | 3.65 | 1.01 | 26.45 | 0.01 | 0.05 | 0.37 | 33.32 | 25.56 | 5.99 | 1.72 |
| Raw HC | 32.77 | 10.40 | 6.06 | 50.74 | 0.04 | 0.32 | 1.55 | - | 18.46 | - | - |
| HC-200 | 41.88 | 8.35 | 5.12 | 44.63 | 0.02 | 0.20 | 1.06 | 57.45 | 19.61 | 4.85 | 0.91 |
| HC-250 | 53.99 | 5.01 | 2.01 | 38.96 | 0.03 | 0.09 | 0.72 | 48.98 | 20.44 | 5.65 | 1.23 |
| HC-300 | 71.99 | 3.02 | 0.55 | 24.41 | 0.03 | 0.04 | 0.34 | 35.89 | 25.86 | 5.98 | 1.55 |
| Sample | Proximate Analysis (wt %) | DTGMax | |||
|---|---|---|---|---|---|
| Water Content | Volatile Matter | Fixed Carbon | Ash | ||
| Raw LFP | 8.07 | 77.88 | 3.38 | 10.67 | 334.78 |
| LFP-200 | 6.78 | 40.67 | 41.54 | 11.01 | 342.37 |
| LFP-250 | 4.25 | 30.32 | 52.23 | 13.20 | 355.77 |
| LFP-300 | 2.89 | 10.78 | 71.55 | 14.78 | 369.89 |
| Raw HC | 9.23 | 76.45 | 2.99 | 11.33 | 324.89 |
| HC-200 | 6.66 | 39.23 | 40.12 | 13.99 | 335.98 |
| HC-250 | 5.89 | 23.32 | 55.87 | 14.92 | 349.90 |
| HC-300 | 2.12 | 11.88 | 69.33 | 16.67 | 357.99 |
| Sample | BET Surface Area (m2/g) | BJH Cumulative Adsorption Surface Area (m2/g) | Total Pore Volume × 10−3 (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| Raw LFP | 3.02 | 1.04 | 0.022 | 0.56 |
| LFP-200 | 9.76 | 8.88 | 0.036 | 3.23 |
| LFP-250 | 13.65 | 11.09 | 0.055 | 4.98 |
| LFP-300 | 18.32 | 15.77 | 0.068 | 8.98 |
| Raw HC | 1.51 | 1.02 | 0.013 | 0.22 |
| HC-200 | 5.98 | 4.77 | 0.021 | 1.23 |
| HC-250 | 7.12 | 5.43 | 0.034 | 2.12 |
| HC-300 | 10.76 | 8.88 | 0.046 | 5.67 |
| Sample | Temperature | Langmuir Isotherm | Freundlich Isotherm | Temkin Isotherm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | qmax (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g) (L/mg)1/n | 1/n | R2 | B | KT (L/mg) | R2 | |
| LFP-300 | 30 | 20.40 | 0.1658 | 0.038 | 0.998 | 4.474 | 0.401 | 0.879 | 3.964 | 4.5815 | 0.935 |
| 40 | 21.27 | 0.2143 | 0.030 | 0.999 | 7.027 | 0.263 | 0.967 | 3.766 | 25.621 | 0.986 | |
| 50 | 22.72 | 0.3135 | 0.021 | 0.998 | 6.461 | 0.324 | 0.907 | 3.781 | 84.576 | 0.978 | |
| HC-300 | 30 | 17.03 | 0.1691 | 0.035 | 0.992 | 1.229 | 0.534 | 0.932 | 4.002 | 3.2861 | 0.989 |
| 40 | 17.54 | 0.2132 | 0.026 | 0.918 | 1.122 | 0.584 | 0.968 | 3.727 | 3.7488 | 0.933 | |
| 50 | 18.59 | 0.3015 | 0.032 | 0.909 | 1.052 | 0.434 | 0.978 | 3.702 | 2.491 | 0.903 | |
| Sample | Temperature, °K | ∆G° (KJ-mol−1) | ∆H° (KJ-mol−1) | ∆S° (JK−1 mol−1) | R2 |
|---|---|---|---|---|---|
| 303 | −4.526 | +25.870 | +0.0702 | 0.9821 | |
| LFP-300 | 323 | −4.009 | |||
| 343 | −3.118 | ||||
| 303 | −4.477 | +23.462 | +0.0624 | 0.981 | |
| HC-300 | 323 | −4.021 | |||
| 343 | −3.219 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, Z.Z.; Krishnan, B.; Sagadevan, S.; Rafique, R.F.; Hamizi, N.A.B.; Abdul Wahab, Y.; Khan, A.A.; Johan, R.B.; Al-douri, Y.; Kazi, S.N.; et al. Effect of Temperature on the Physical, Electro-Chemical and Adsorption Properties of Carbon Micro-Spheres Using Hydrothermal Carbonization Process. Nanomaterials 2018, 8, 597. https://doi.org/10.3390/nano8080597
Chowdhury ZZ, Krishnan B, Sagadevan S, Rafique RF, Hamizi NAB, Abdul Wahab Y, Khan AA, Johan RB, Al-douri Y, Kazi SN, et al. Effect of Temperature on the Physical, Electro-Chemical and Adsorption Properties of Carbon Micro-Spheres Using Hydrothermal Carbonization Process. Nanomaterials. 2018; 8(8):597. https://doi.org/10.3390/nano8080597
Chicago/Turabian StyleChowdhury, Zaira Zaman, Bagavathi Krishnan, Suresh Sagadevan, Rahman Faizur Rafique, Nor Aliya Binti Hamizi, Yasmin Abdul Wahab, Ali Akbar Khan, Rafie Bin Johan, Y. Al-douri, Salim Newaz Kazi, and et al. 2018. "Effect of Temperature on the Physical, Electro-Chemical and Adsorption Properties of Carbon Micro-Spheres Using Hydrothermal Carbonization Process" Nanomaterials 8, no. 8: 597. https://doi.org/10.3390/nano8080597