Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts
Abstract
:1. Introduction
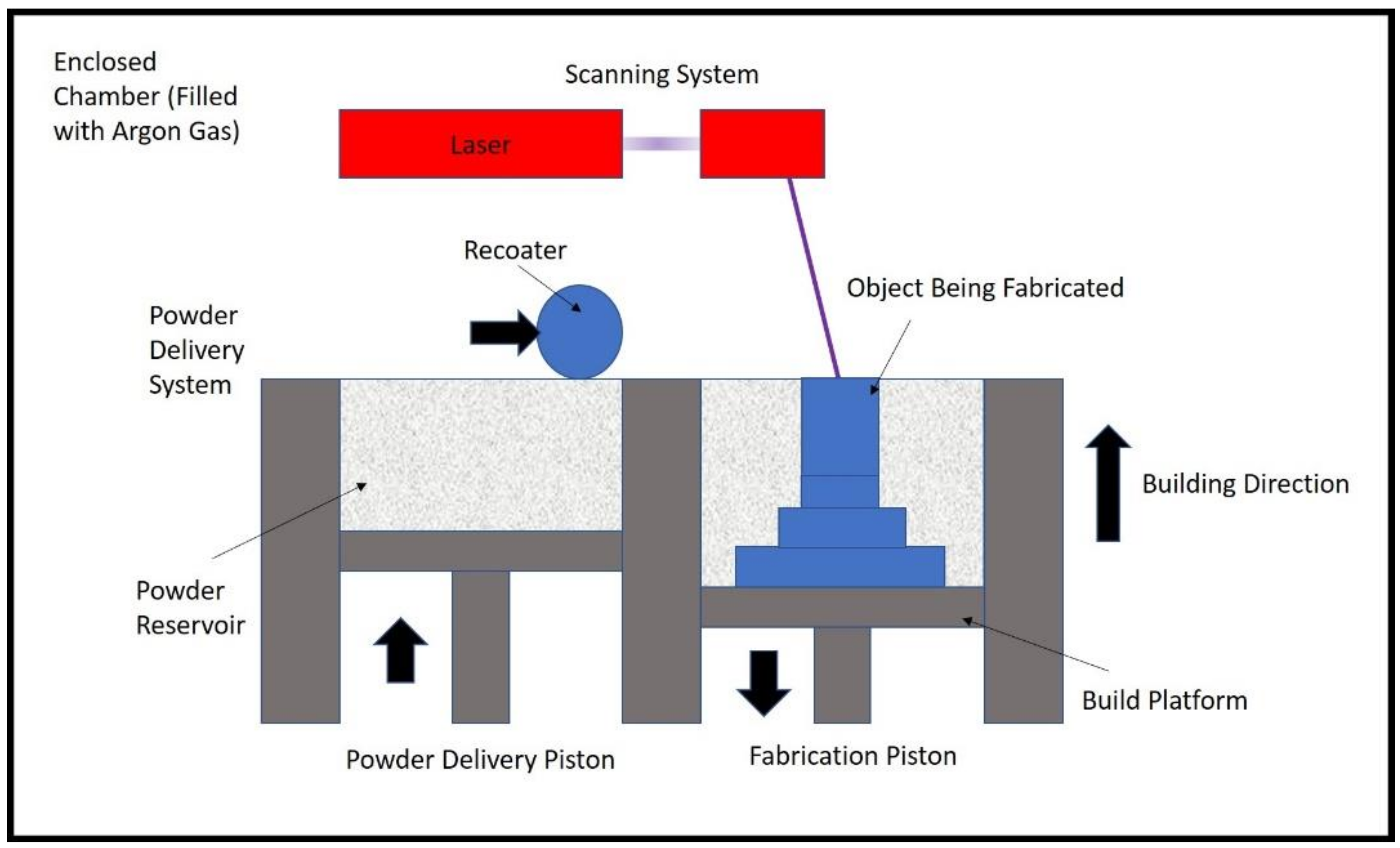
2. Experiment
3. Results and Discussion
3.1. Partially Melted Powder
3.2. Laser Induced Periodical Surface Structures
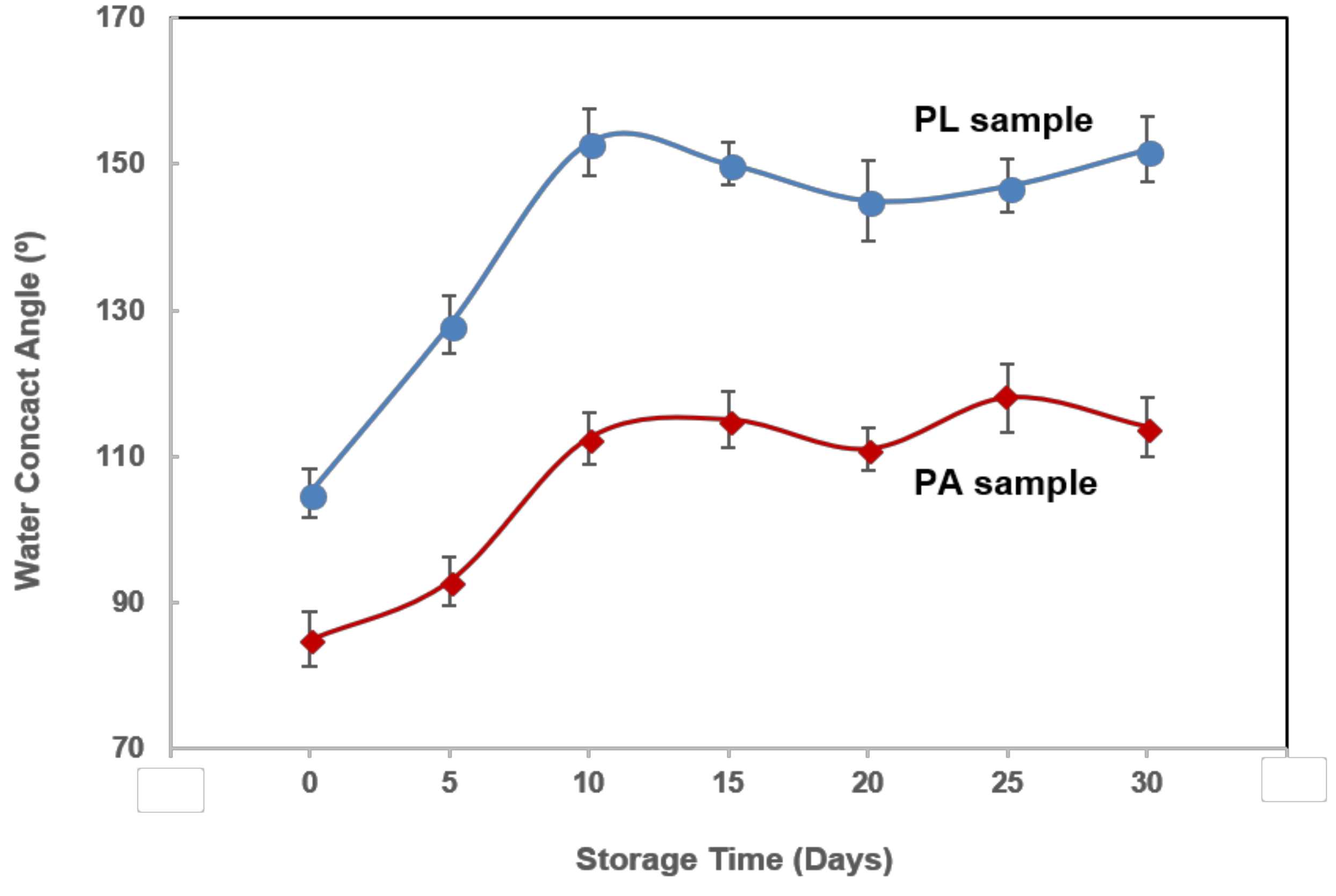
3.3. Wettability
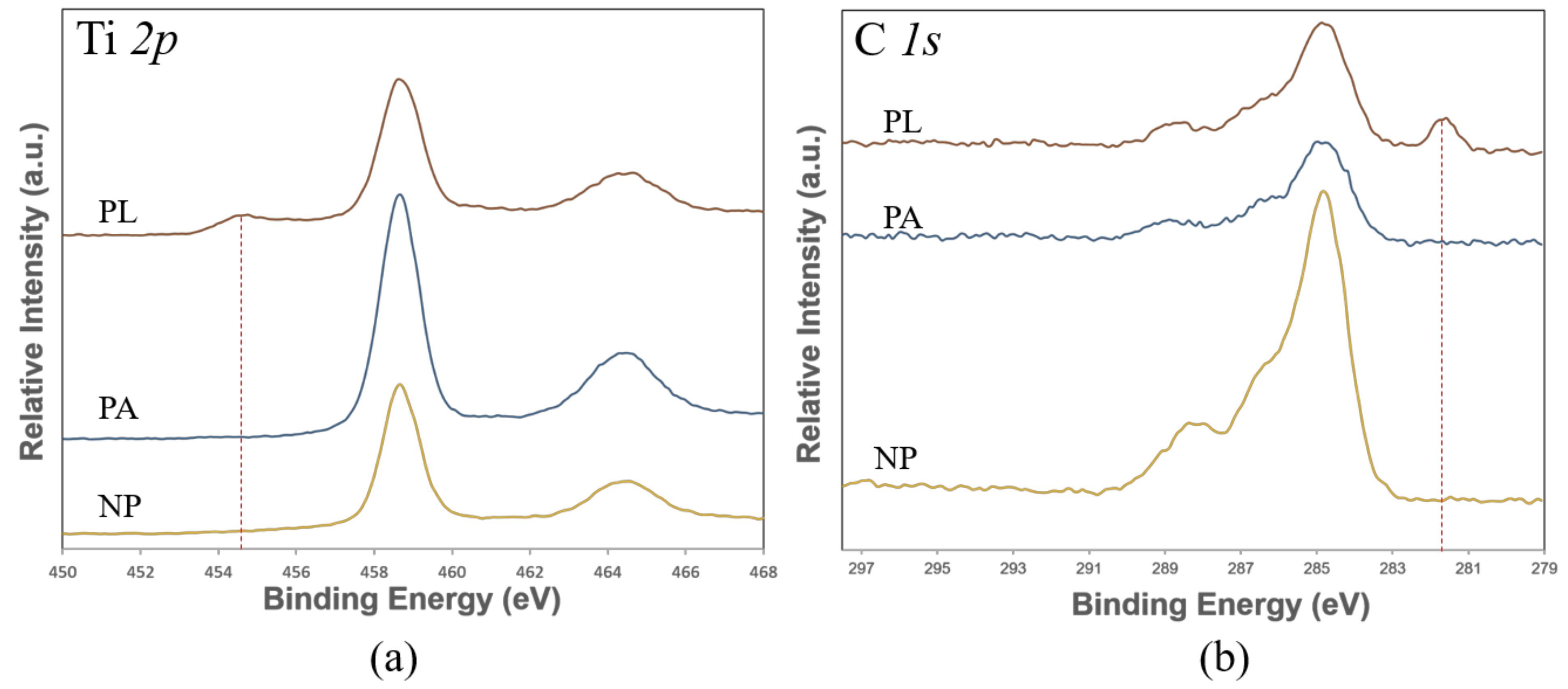
3.4. Chemical Composition
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bhaduri, D.; Penchev, P.; Batal, A.; Dimov, S.; Soo, S.L.; Sten, S.; Harrysson, U.; Zhang, Z.; Dong, H. Laser polishing of 3D printed mesoscale components. Appl. Surf. Sci. 2017, 405, 29–46. [Google Scholar] [CrossRef]
- Huang, Y.; Leu, M.C.; Mazumder, J.; Donmez, A. Additive manufacturing: Current state, future potential, gaps and needs, and recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Q.; Cheng, L.; Li, S.; Shi, Y. Effects of scan line spacing on pore characteristics and mechanical properties of porous Ti6AL4V implants fabricated by selective laser melting. Mater. Des. 2014, 63, 185–193. [Google Scholar] [CrossRef]
- Wieding, J.; Jonitz, A.; Bader, R. The effect of structural design on mechanical properties and cellular response of additive manufactured titanium scaffolds. Materials 2012, 5, 1336–1347. [Google Scholar] [CrossRef]
- Turner, B.N.; Gold, S.A. A review of melt extrusion additive manufacturing processes: II. Materials, dimensional accuracy, and surface roughness. Rapid Prototyp. J. 2015, 21, 250–261. [Google Scholar] [CrossRef]
- Xiang, T.; Ding, S.; Li, C.; Zheng, S.; Hu, W.; Wang, J.; Liu, P. Effect of current density on wettability and corrosion resistance of superhydrophobic nickel coating deposited on low carbon steel. Mater. Des. 2017, 114, 65–72. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, Y.; Liu, A.; Zhu, Y.; Liu, S.; Jiang, H. Bio-inspiredly fabricating the hierarchical 3D porous structure superhydrophobic surfaces for corrosion prevention. Mater. Des. 2016, 103, 300–307. [Google Scholar] [CrossRef]
- Hwang, S.J.; Tseng, M.C.; Shu, J.R.; Yu, H.H. Surface modification of cyclic olefin copolymer substrate by oxygen plasma treatment. Surf. Coat. Technol. 2008, 202, 3669–3674. [Google Scholar] [CrossRef]
- Liu, T.; Yin, Y.; Chen, S.; Chang, X.; Cheng, S. Super-hydrophobic surfaces improve corrosion resistance of copper in seawater. Electrochim. Acta 2007, 52, 3709–3713. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Wang, Y.; Han, Z.; Ren, L. Biomimetic hydrophobic surface fabricated by chemical etching method from hierarchically structured magnesium alloy substrate. Appl. Surf. Sci. 2013, 280, 845–849. [Google Scholar] [CrossRef]
- Skoulas, E.; Manousaki, A.; Fotakis, C.; Stratakis, E. Biomimetic surface structuring using cylindrical vector femtosecond laser beams. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, L.; Zhang, Z.; Wu, S.; Li, G.; Wu, P.; Hu, Y.; Li, J.; Chu, J.; Wu, D. Biomimetic surfaces with anisotropic sliding wetting by energy-modulation femtosecond laser irradiation for enhanced water collection. RSC Adv. 2017, 7, 11170–11179. [Google Scholar] [CrossRef] [Green Version]
- George, J.E.; Rodrigues, V.R.M.; Mathur, D.; Chidangil, S.; George, S.D. Self-cleaning superhydrophobic surfaces with underwater superaerophobicity. Mater. Des. 2016, 100, 8–18. [Google Scholar] [CrossRef]
- Shugaev, M.V.; Wu, C.; Armbruster, O.; Naghilou, A.; Brouwer, N.; Ivanov, D.S.; Derrien, T.J.Y.; Bulgakova, N.M.; Kautek, W.; Rethfeld, B.; et al. Fundamentals of ultrafast laser–material interaction. MRS Bull. 2016, 41, 960–968. [Google Scholar] [CrossRef]
- Stratakis, E.; Ranella, A.; Fotakis, C. Biomimetic micro/nanostructured functional surfaces for microfluidic and tissue engineering applications. Biomicrofluidics 2011, 5, 013411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.P.; Guan, Y.C.; Zhou, W. Laser polishing of additive manufactured ti alloys. Opt. Lasers Eng. 2017, 93, 171–177. [Google Scholar] [CrossRef]
- Meier, H.; Haberland, C. Experimental studies on selective laser melting of metallic parts. Mater. Werkst. 2008, 39, 665–670. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Goodridge, R.D.; Hague, R.J.M.; Christie, S.D.R.; Edmondson, S. The effect of laser remelting on the surface chemistry of Ti6Al4V components fabricated by selective laser melting. J. Mater. Process. Technol. 2016, 232, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bonch-Bruevich, A.M.; Libenson, M.N.; Makin, V.S.; Trubaev, V.V. Surface Electromagnetic Waves in Optics; SPIE: Bellingham, WA, USA, 1992; p. 13. [Google Scholar]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti-6AL-4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef]
- Jiao, L.S.; Ng, E.Y.K.; Zheng, H.Y. Refining femtosecond laser induced periodical surface structures with liquid assist. Appl. Surf. Sci. 2013, 264, 52–55. [Google Scholar] [CrossRef]
- Bonse, J.; Koter, R.; Hartelt, M.; Spaltmann, D.; Pentzien, S.; Höhm, S.; Rosenfeld, A.; Krüger, J. Tribological performance of femtosecond laser-induced periodic surface structures on titanium and a high toughness bearing steel. Appl. Surf. Sci. 2015, 336, 21–27. [Google Scholar] [CrossRef]
- Kröger, E.; Kretschmann, E. Surface plasmon and polariton dispersion at rough boundaries. Phys. Status Solidi (b) 1976, 76, 515–523. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Makin, V.S.; Guo, C. Periodic ordering of random surface nanostructures induced by femtosecond laser pulses on metals. J. Appl. Phys. 2007, 101, 034903. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.C.; Zheng, H.Y.; Lam, Y.C. Femtosecond laser-induced surface wettability modification of polystyrene surface. Sci. China Phys. Mech. Astron. 2016, 59, 124211. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Jiao, L.S.; Ng, E.Y.K.; Wee, L.M.; Zheng, H.Y. Role of volatile liquids in debris and hole taper angle reduction during femtosecond laser drilling of silicon. Appl. Phys. A Mater. Sci. Process. 2011, 104, 1081–1084. [Google Scholar] [CrossRef]
- Yang, C.-J.; Mei, X.-S.; Tian, Y.-L.; Zhang, D.-W.; Li, Y.; Liu, X.-P. Modification of wettability property of titanium by laser texturing. Int. J. Adv. Manuf. Technol. 2016, 87, 1663–1670. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, H.; Shioyama, T.; Suda, Y.; Yamanaka, M.; Kannari, F.; Itakura, R.; Yamanouchi, K. Controlling the dissociative ionization of ethanol with 800 and 400 nm two-color femtosecond laser pulses. J. Chem. Phys. 2007, 127. [Google Scholar] [CrossRef] [PubMed]
- Bizi-bandoki, P.; Valette, S.; Audouard, E.; Benayoun, S. Time dependency of the hydrophilicity and hydrophobicity of metallic alloys subjected to femtosecond laser irradiations. Appl. Surf. Sci. 2013, 273, 399–407. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Domantovsky, A.G.; Shiryaev, A.A. Comment on “nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications” by duong v. Ta, andrew dunn, thomas j. Wasley, robert w. Kay, jonathan stringer, patrick j. Smith, colm connaughton, jonathan d. Shephard (Appl. Surf. Sci. 357 (2015) 248–254). Appl. Surf. Sci. 2016, 379, 111–113. [Google Scholar]



| Parameters | Unit | Values |
|---|---|---|
| Laser Power | W | 95 |
| Layer Thickness | mm | 0.050 |
| Scan Speed | mm/s | 125 |
| Hatch space | mm | 0.11 |
| Parameters | Unit | Values |
|---|---|---|
| Scan Speed | mm/s | 20, 40, 80 |
| Hatch density | mm | 0.02 |
| Laser spot size | µm | 120 |
| Laser intensity | W/cm2 | 4.9 × 1013 |
| Sample Name | Relative Atomic Percentage of Detected Elements | |||||
|---|---|---|---|---|---|---|
| Al 2p | C 1s | N 1s | O 1s | Ti 2p | V 2p | |
| Non-processed (NP) | 3.86 | 36.75 | 4.41 | 42.96 | 12.01 | 0.30 |
| Processed in air (PA) | 5.58 | 14.95 | 1.23 | 54.89 | 23.35 | 0.21 |
| Processed in liquid (PL) | 4.09 | 23.94 | 1.64 | 50.49 | 19.84 | 0.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.; Chua, Z.Y.; Moon, S.K.; Song, J.; Bi, G.; Zheng, H. Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts. Nanomaterials 2018, 8, 601. https://doi.org/10.3390/nano8080601
Jiao L, Chua ZY, Moon SK, Song J, Bi G, Zheng H. Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts. Nanomaterials. 2018; 8(8):601. https://doi.org/10.3390/nano8080601
Chicago/Turabian StyleJiao, Lishi, Zhong Yang Chua, Seung Ki Moon, Jie Song, Guijun Bi, and Hongyu Zheng. 2018. "Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts" Nanomaterials 8, no. 8: 601. https://doi.org/10.3390/nano8080601






