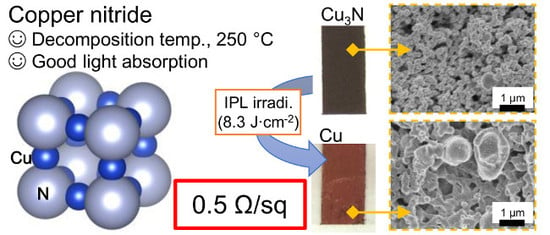
Suitability of Copper Nitride as a Wiring Ink Sintered by Low-Energy Intense Pulsed Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Materials for Comparison with Copper Nitride
2.1.2. Materials for Preparation of Copper Nitride Nanoparticles
2.1.3. Materials for Preparation of Vehicles for Liquid Inks
2.1.4. Materials for Preparation of Vehicles for Paste Inks
2.2. Preparation of Copper Nitride Nanoparticles
2.3. Preparation of Liquid Inks
2.4. Synthesis of Poly(3-glycidoxypropyl)trimethoxysilane as an Adhesion Compound
2.5. Preparation of Paste Inks
2.6. Intense Pulsed Light Sintering
2.7. Characterization of Copper Compounds and Inks
2.8. Calculation of Copper Conversion Ratio
3. Results and Discussion
3.1. Comparison of the Light Absorption and Thermal Decomposition Properties of the Copper Compounds
3.2. Evaluation of Liquid Inks
3.3. Evaluation of Paste Inks
3.3.1. Use of Ethyl Cellulose and PGPTMS as a Binder and an Adhesion Reagent, respectively, to Produce a Copper Nitride Paste Ink
3.3.2. Comparison of Paste Inks Prepared with Vehicle 2
3.4. Volume Resistivity and Comparison with that of Previous Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wünscher, S.; Abbel, R.; Perelaer, J.; Schubert, U.S. Progress of alternative sintering approaches of inkjet-printed metal inks and their application for manufacturing of flexible electronic devices. J. Mater. Chem. C 2014, 2, 10232–10261. [Google Scholar] [CrossRef]
- Perelaer, J.; Smith, P.J.; Mager, D.; Soltman, D.; Volkman, S.K.; Subramanian, V.; Korvink, J.G.; Schubert, U.S. Printed electronics: The challenges involved in printing devices, interconnects, and contacts based on inorganic materials. J. Mater. Chem. 2010, 20, 8446–8453. [Google Scholar] [CrossRef]
- Perelaer, J.; Schubert, U.S. Novel approaches for low temperature sintering of inkjet-printed inorganic nanoparticles for roll-to-roll (R2R) applications. J. Mater. Res. 2013, 28, 564–573. [Google Scholar] [CrossRef]
- Singh, R.; Singh, E.; Nalwa, H.S. Inkjet printed nanomaterial based flexible radio frequency identification (RFID) tag sensors for the internet of nano things. RSC Adv. 2017, 7, 48597–48630. [Google Scholar] [CrossRef] [Green Version]
- Perelaer, J.; Jani, R.; Grouchko, M.; Kamyshny, A.; Magdassi, S.; Schubert, U.S. Plasma and microwave flash sintering of a tailored silver nanoparticle ink, yielding 60% bulk conductivity on cost- effective polymer foils. Adv. Mater. 2012, 24, 3993–3998. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Oh, K.H.; Kim, H.S. All-photonic drying and sintering process via flash white light combined with deep-UV and near-infrared irradiation for highly conductive copper nano-ink. Sci. Rep. 2016, 6, 19696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Rovere, T.; Weerawarne, D.; Osterhoudt, G.; Kang, N.; Joseph, P.; Luo, J.; Shim, B.; Poliks, M.; Zhong, C.-J. Nanoalloy printed and pulse-laser sintered flexible sensor devices with enhanced stability and materials compatibility. ACS Nano 2015, 9, 6168–6177. [Google Scholar] [CrossRef] [PubMed]
- Zenou, M.; Ermak, O.; Saar, A.; Kotler, Z. Laser sintering of copper nanoparticles. J. Phys. D 2014, 47, 25501. [Google Scholar] [CrossRef]
- Kim, H.S.; Dhage, S.R.; Shim, D.E.; Hahn, H.T. Intense pulsed light sintering of copper nanoink for printed electronics. Appl. Phys. A 2009, 97, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Dharmadasa, R.; Jha, M.; Amos, D.A.; Druffel, T. Room temperature synthesis of a copper ink for the intense pulsed light sintering of conductive copper films. ACS Appl. Mater. Interfaces 2013, 5, 13227–13234. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Yoo, T.H.; Song, Y.W.; Lim, D.S.; Oh, Y.J. Cu ion ink for a flexible substrate and highly conductive patterning by intensive pulsed light sintering. ACS Appl. Mater. Interfaces 2013, 5, 4113–4119. [Google Scholar] [CrossRef] [PubMed]
- Han, W.S.; Hong, J.M.; Kim, H.S.; Song, Y.W. Multi-pulsed white light sintering of printed Cu nanoinks. Nanotechnology 2011, 22, 395705. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.; Dharmadasa, R.; Draper, G.L.; Sherehiy, A.; Sumanasekera, G.; Amos, D.; Druffel, T. Solution phase synthesis and intense pulsed light sintering and reduction of a copper oxide ink with an encapsulating nickel oxide barrier. Nanotechnology 2015, 26, 175601. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, A.; Rivadeneyra, A.; Abdellah, A.; Lugli, P.; Salmerón, J.F. Inkjet printing and photonic sintering of silver and copper oxide nanoparticles for ultra-low-cost conductive patterns. J. Mater. Chem. C 2016, 4, 3546–3554. [Google Scholar] [CrossRef]
- Kang, H.; Sowade, E.; Baumann, R.R. Direct intense pulsed light sintering of inkjet-printed copper oxide layers within six milliseconds. ACS Appl. Mater. Interfaces 2014, 6, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Hwang, H.J.; Lee, S.H.; Kim, H.S. In situ monitoring of a flash light sintering process using silver nano-ink for producing flexible electronics. Nanotechnology 2013, 24, 35202. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Hwang, Y.T.; Lee, S.H.; Kim, H.S. Electrical wire explosion process of copper/silver hybrid nano-particle ink and its sintering via flash white light to achieve high electrical conductivity. Nanotechnology 2016, 27, 205704. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J.; Hwang, H.J.; Kim, H.S. Highly conductive copper nano/microparticles ink via flash light sintering for printed electronics. Nanotechnology 2014, 25, 265601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, T.; Chen, M.; Xiao, F. Mixed ink of copper nanoparticles and copper formate complex with low sintering temperatures. J. Mater. Sci. 2016, 27, 11432–11438. [Google Scholar] [CrossRef]
- Yang, W.D.; Liu, C.Y.; Zhang, Z.Y.; Liu, Y.; Nie, S.D. Copper inks formed using short carbon chain organic Cu-precursors. RSC Adv. 2014, 4, 60144–60147. [Google Scholar] [CrossRef]
- Yang, W.D.; Wang, C.H.; Arrighi, V.; Liu, C.Y.; Watson, D. Microstructure and electrical property of copper films on a flexible substrate formed by an organic ink with 9.6% of Cu content. J. Mater. Sci. 2015, 26, 8973–8982. [Google Scholar] [CrossRef]
- Santos, G.M.C. Cu2O Nanoparticles for Application in Printed and Flexible Electronics. Ph.D. Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Nakamura, T.; Katayama, M.; Watanabe, T.; Inada, Y.; Ebina, T.; Yamaguchi, A. Stability of copper nitride nanoparticles under high humidity and in solutions with different acidity. Chem. Lett. 2015, 44, 755–757. [Google Scholar] [CrossRef]
- Nakamura, T.; Hayashi, H.; Hanaoka, T.; Ebina, T. Preparation of copper nitride (Cu3N) nanoparticles in long-chain alcohols at 130–200 °C and nitridation mechanism. Inorg. Chem. 2014, 53, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Juza, R.; Hahn, H. Crystal structures of Cu3N, GaN and InN-metallic amides and metallic nitrides. Z. Anorg. Allg. Chem. 1938, 239, 282–287. [Google Scholar] [CrossRef]
- Fan, X.Y.; Li, Z.J.; Meng, A.; Li, C.; Wu, Z.G.; Yan, P.X. Improving the thermal stability of Cu3N films by addition of mn. J. Mater. Sci. Technol. 2015, 31, 822–827. [Google Scholar] [CrossRef]
- Nakamura, T.; Hayashi, H.; Ebina, T. Preparation of copper nitride nanoparticles using urea as a nitrogen source in a long-chain alcohol. J. Nanopart. Res. 2014, 16, 2699. [Google Scholar] [CrossRef]
- Hong, J.U.; Kumar, A.; Han, H.S.; Koo, Y.H.; Kim, H.W.; Park, J.H.; Kang, H.S.; Lee, B.C.; Piao, L.; Kim, S.H. Alkoxysilane adhesion promoter for Ag nano-ink. Bull. Korean Chem. Soc. 2013, 34, 2539–2542. [Google Scholar] [CrossRef]
- Jiang, J.; Koo, Y.H.; Kim, H.W.; Park, J.H.; Kang, H.S.; Lee, B.C.; Kim, S.H.; Song, H.E.; Piao, L. High-temperature adhesion promoter based on (3-glycidoxypropyl) trimethoxysilane for Cu paste. Bull. Korean Chem. Soc. 2014, 35, 3025–3029. [Google Scholar] [CrossRef]
- Predel, B. Cu–O (copper-oxygen). In Cr–Cs–Cu–Zr; Madelung, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 5D. [Google Scholar]
- Hwang, H.J.; Chung, W.H.; Kim, H.S. In situ monitoring of flash-light sintering of copper nanoparticle ink for printed electronics. Nanotechnology 2012, 23, 485205. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ryu, C.H.; Park, S.H.; Kim, H.S. The effect of poly (N-vinylpyrrolidone) molecular weight on flash light sintering of copper nanopaste. Thin Solid Films 2014, 570, 114–122. [Google Scholar] [CrossRef]
- Joo, S.J.; Park, S.H.; Moon, C.J.; Kim, H.S. A highly reliable copper nanowire/nanoparticle ink pattern with high conductivity on flexible substrate prepared via a flash light-sintering technique. ACS Appl. Mater. Interfaces 2015, 7, 5674–5684. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; James, R.; Kell, A.J.; Mozenson, O.; Ferrigno, J.; Lafreniere, S.; Malenfant, P.R.L. Photosintering and electrical performance of CuO nanoparticle inks. Org. Electron. 2014, 15, 1836–1842. [Google Scholar] [CrossRef]







| Copper-Containing Material | Light Absorbency | Decomposition Temperature or Melting Point (°C) | Copper Content Ratio | |
|---|---|---|---|---|
| Absorption Range (nm) | Strength | |||
| Cu3N | 350–800 | Medium | 250 | 93 |
| CuO | 350–800 | Strong | 1193 | 87 |
| Cu2O | 350–650 | Medium | 1229 | 89 |
| Cu | 350–800 | Weak | 1085 | 100 |
| Energy Density (J cm−2) | Vehicle | Conversion Ratio | Sheet Resistance (Ω sq−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Cu3N | CuO | Cu2O | Cu3N | CuO | Cu2O | Cu | ||
| 12.45 | EtOH | 0.91 | 0 | 0 | 4.52 × 106 | N.D. | O.L. | 2.70 × 10−1 |
| 16.60 | EtOH | 0.88 | 0 | 0 | 2.37 × 100 | N.D. | O.L. | 1.11 × 10−1 |
| 12.45 | EtOH + EG | 0.99 | 0 | 0 | 1.34 × 100 | N.D. | O.L. | 3.31 × 10−1 |
| 16.60 | EtOH + EG | 0.96 | 0 | 0 | 6.95 × 10−1 | N.D. | O.L. | 4.72 × 10−1 |
| Type of Vehicle | PGPTMS (wt %) | 2-(2-Butoxyethoxy)ethyl acetate (wt %) | Diethylene Glycol Monobutyl Ether (wt %) | Ethyl Cellulose (wt %) |
|---|---|---|---|---|
| Vehicle 1 | 0 | 83.7 | 9.3 | 7 |
| Vehicle 2 | 1 | 82.9 | 9.2 | 6.9 |
| Vehicle 3 | 7 | 77.9 | 8.7 | 6.5 |
| Photo Sintering Condition | Applied Voltage (kV) | Pulse Width (μs) | Period (ms) | Number of Pulses | Total Electrical Energy (J) | Energy Density (J cm−2) | Distance (mm) |
|---|---|---|---|---|---|---|---|
| P.S.1 | 2.0 | 1000 | 1000 | 1 | 344.0 | 5.94 | 25 |
| P.S.2 | 2.3 | 1000 | 1000 | 1 | 481.0 | 8.30 | 25 |
| P.S.3 | 2.3 | 1500 | 1000 | 1 | 721.6 | 12.45 | 25 |
| P.S.4 | 2.3 | 2000 | 1000 | 1 | 962.1 | 16.60 | 25 |
| P.S.5 | 2.3 | 1000 | 1000 | 4 | 481.0 | 8.30 | 25 |
| IPL Sintering Condition | Conversion Ratio | Sheet Resistance (Ω sq−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu3N | CuO | Cu2O | Cu3N | CuO | Cu2O | |||||
| Vehicle | 1 | 2 | 3 | 2 | 2 | 1 | 2 | 3 | 2 | 2 |
| P.S.1 | 0.10 | 0.43 | 0.35 | 0.00 | 0.00 | O.L. | 2.74 × 100 | O.L. | O.L. | O.L. |
| P.S.2 | 0.41 | 0.63 | 0.68 | 0.00 | 0.00 | O.L. | 5.06 × 10−1 | O.L. | O.L. | O.L. |
| P.S.3 | 0.62 | 0.84 | 0.67 | 0.03 | 0.00 | 9.65 × 100 | 1.19 × 100 | 9.65 × 100 | O.L. | O.L. |
| P.S.4 | 0.69 | 0.82 | 0.62 | 0.10 | 0.00 | 1.41 × 100 | 7.90 × 100 | 2.82 × 100 | O.L. | O.L. |
| P.S.5 | 0.46 | 0.75 | 0.56 | 0.00 | 0.00 | O.L. | 4.97 × 10−1 | O.L. | O.L. | O.L. |
| Entry | Compound | Form | Lower IPL Irradiation Energy | Higher IPL Irradiation Energy | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Energy Density (J cm−2) | Volume Resistivity (μΩ cm) | Sheet Resistance (mΩ sq−1) | Energy Density (J cm−2) | Volume Resistivity (μΩ cm) | Sheet Resistance (mΩ sq−1) | ||||
| 1 | Copper nitride | Nanoparticle | 8.3 | 607 | 506 | – | – | – | This work |
| 2 | Copper | Nanoparticle | 20 | Infi. | Infi. | 45 | 5 | – | [9] |
| 3 | Copper | Nanoparticle | 12 | Infi. | Infi. | 32 | 173 | – | [12] |
| 4 | Copper | Nanoparticle | 10 | – | 9860 | 12.5 | – | 72 | [31] |
| 5 | Copper | Nanoparticle | 8.0 | 450 | – | 12.5 | 54 | – | [32] |
| 6 | Copper | Nanoparticle + microparticle | 10.0 | 438 | – | 12.5 | 80 | – | [18] |
| 7 | Copper | Nanoparticle + nanowire | 7.5 | 420–520 | – | 12.5 | 22.7 | – | [33] |
| 8 | Copper(II) oxide | – | 10.9 | – | 200 | 11 | – | 170 | [34] |
| 9 | Copper organic complex | – | 16.5 | 750 | – | 40.6 | 4.6 | – | [11] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T.; Cheong, H.J.; Takamura, M.; Yoshida, M.; Uemura, S. Suitability of Copper Nitride as a Wiring Ink Sintered by Low-Energy Intense Pulsed Light Irradiation. Nanomaterials 2018, 8, 617. https://doi.org/10.3390/nano8080617
Nakamura T, Cheong HJ, Takamura M, Yoshida M, Uemura S. Suitability of Copper Nitride as a Wiring Ink Sintered by Low-Energy Intense Pulsed Light Irradiation. Nanomaterials. 2018; 8(8):617. https://doi.org/10.3390/nano8080617
Chicago/Turabian StyleNakamura, Takashi, Hea Jeong Cheong, Masahiko Takamura, Manabu Yoshida, and Sei Uemura. 2018. "Suitability of Copper Nitride as a Wiring Ink Sintered by Low-Energy Intense Pulsed Light Irradiation" Nanomaterials 8, no. 8: 617. https://doi.org/10.3390/nano8080617