Synthesis of Amorphous Carbon Film in Ethanol Inverse Diffusion Flames
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental System
2.2. Experimental Conditions
2.3. Characterizations and Transfer
3. Results and Discussion
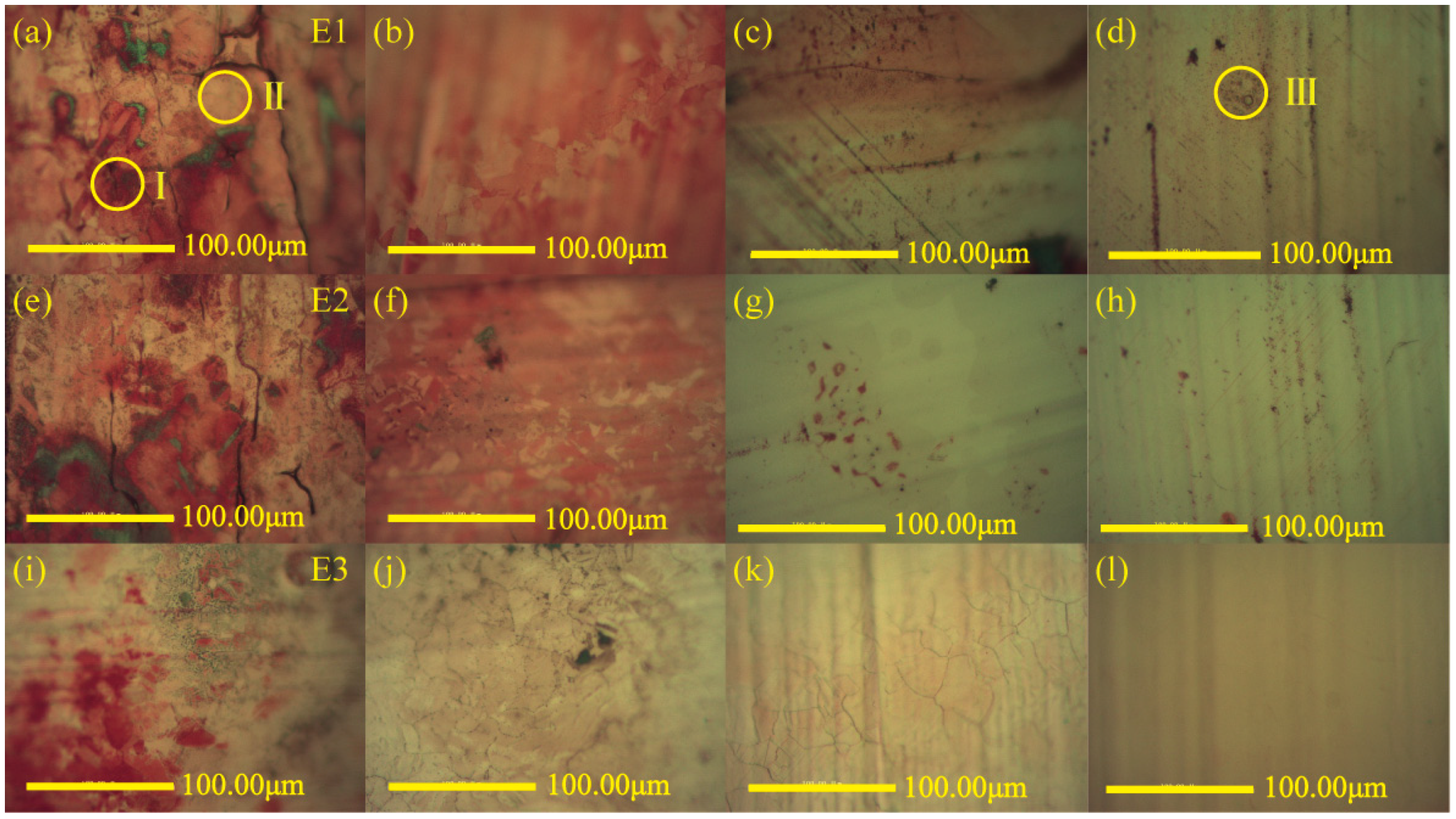
3.1. Growth of Carbon Films on Copper Foils
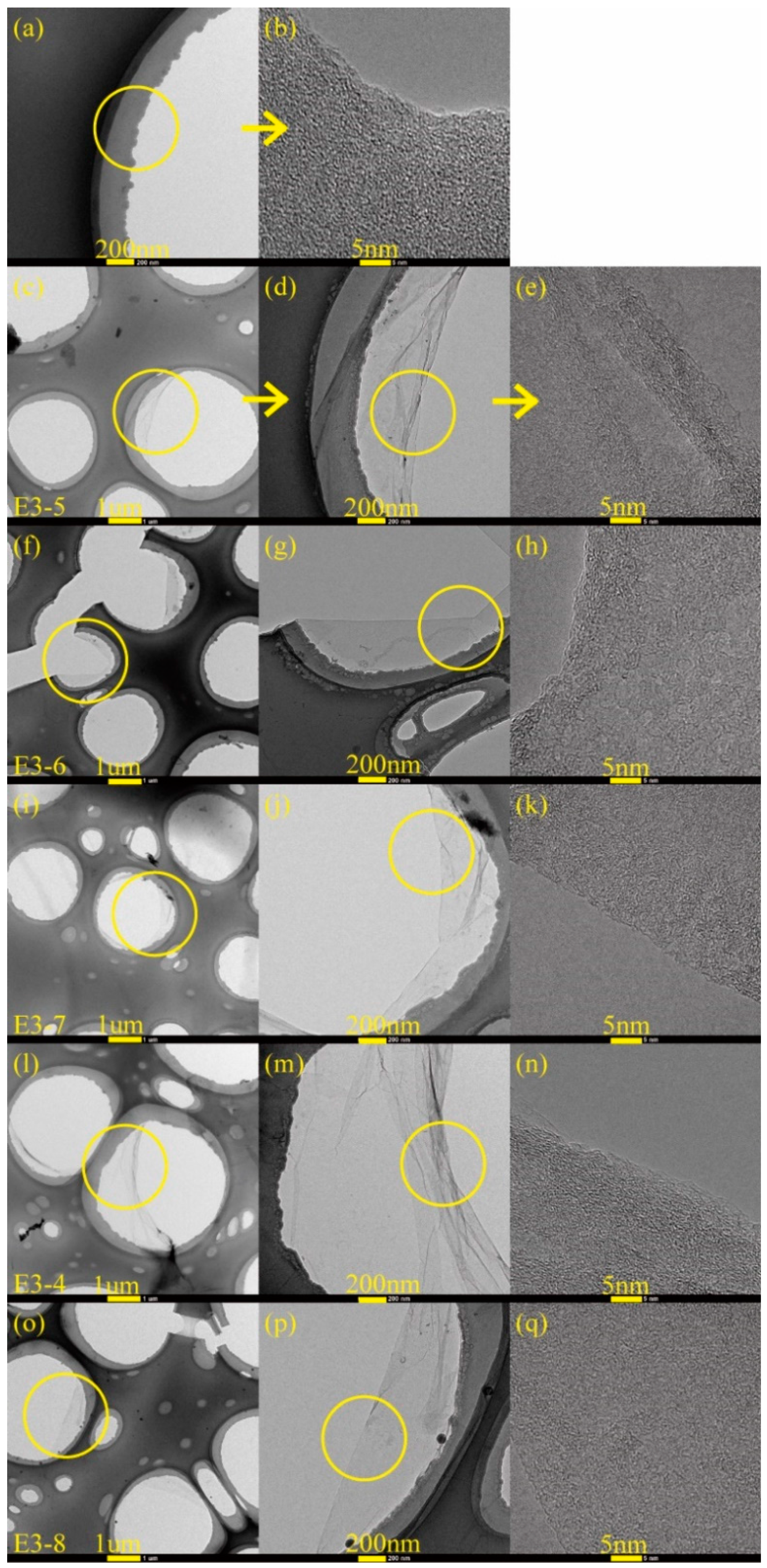
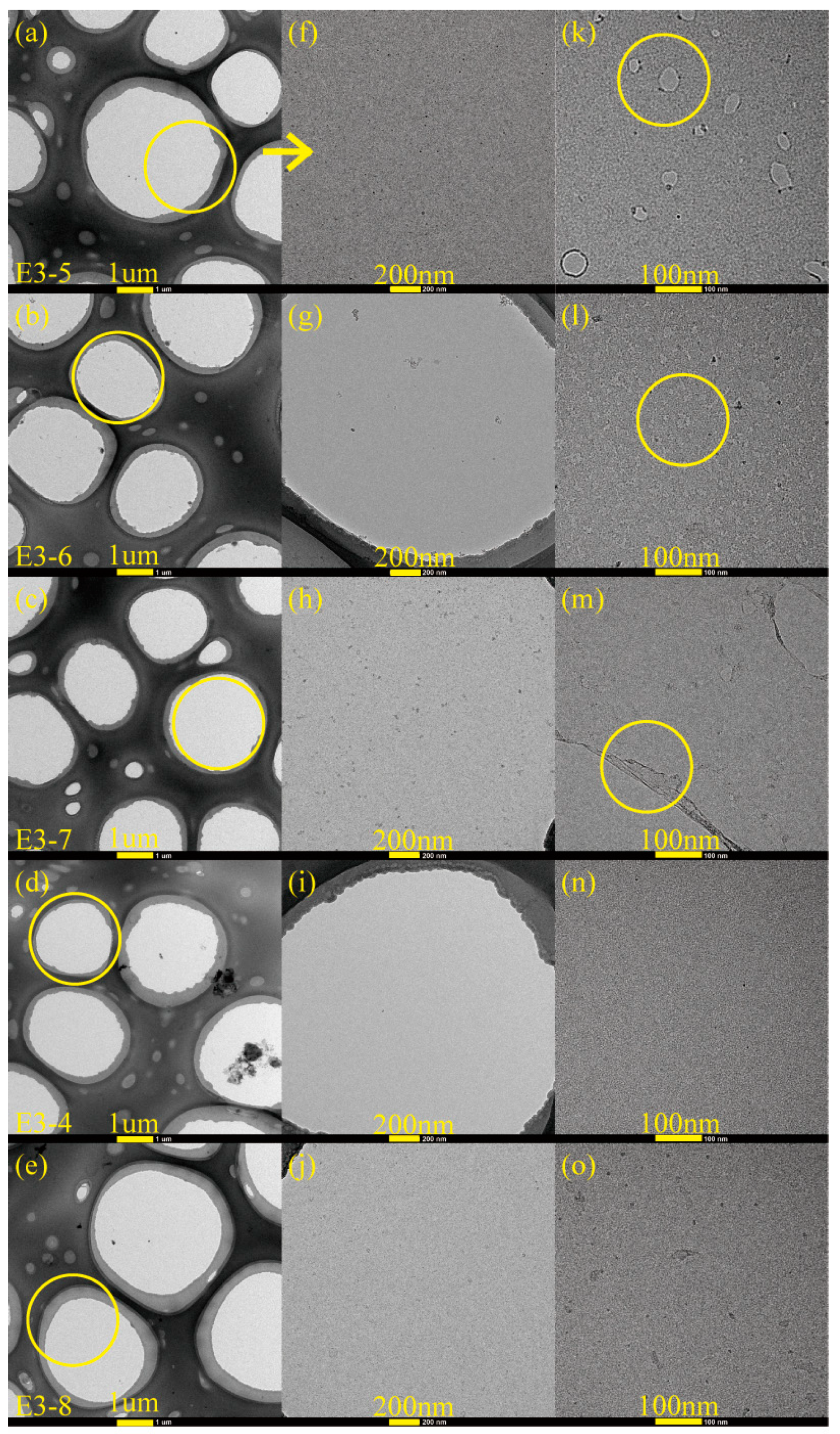
3.2. Microstructure Analysis of Carbon Films
3.3. Raman Spectra and XRD Analysis
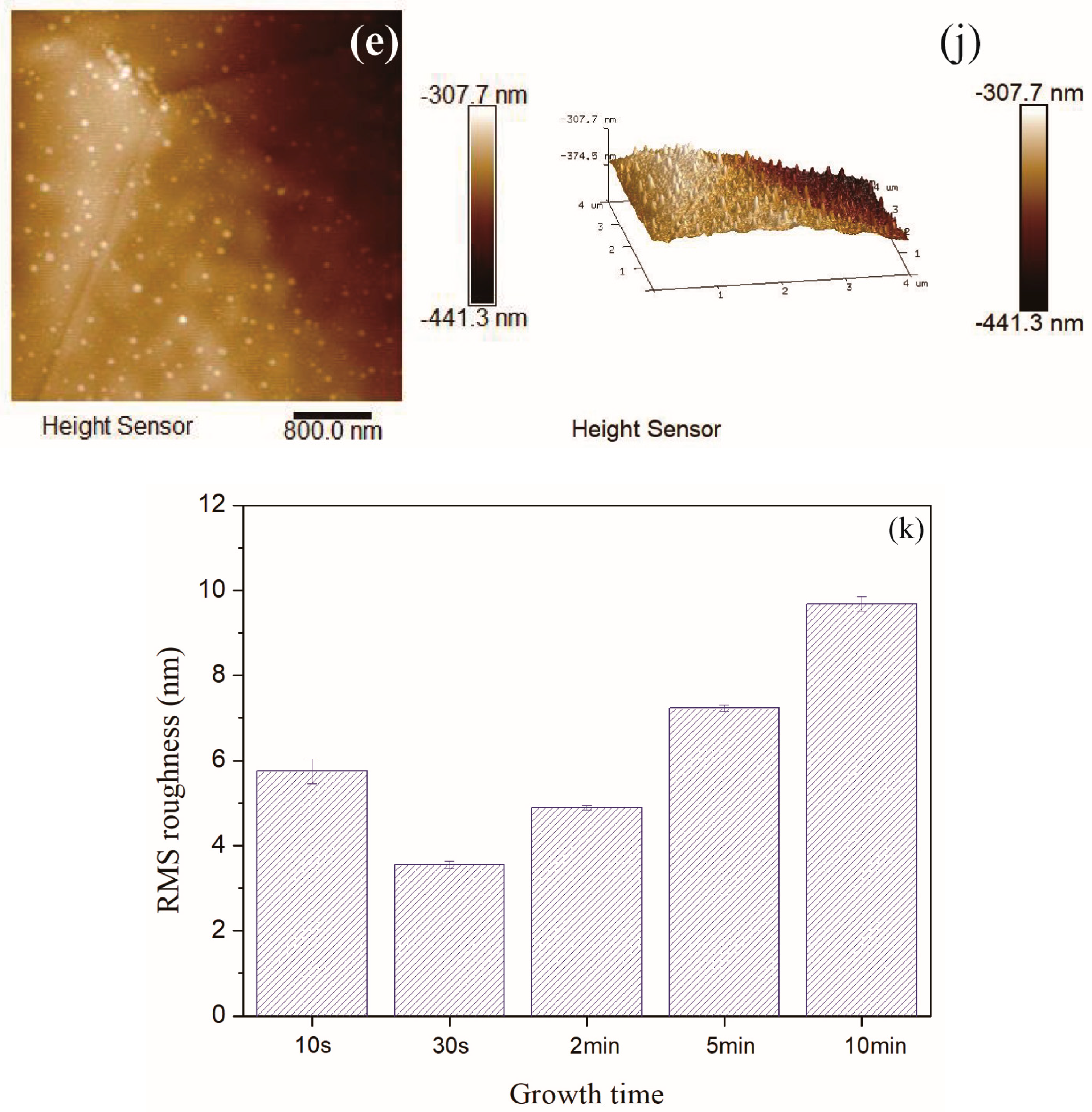
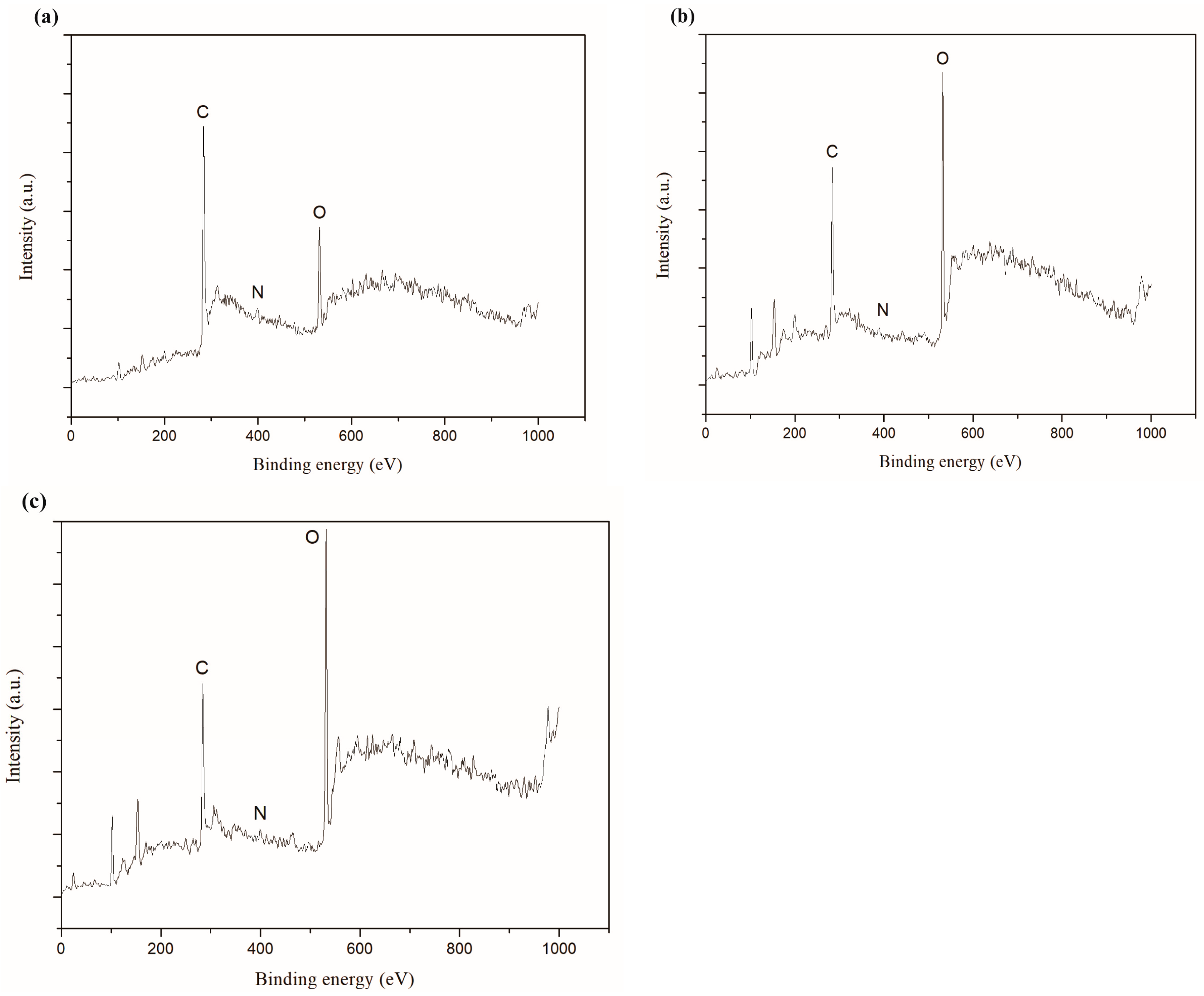
3.4. AFM Images Analysiss, Film Thickness and Elemental Composition
4. Conclusions
- (1)
- In open-atmosphere environments, utilizing inverse diffusion flame, with ethanol as fuel and copper foil as synthesis substrate, amorphous carbon film emerged at only 10 s. The synthetic carbon film was ultra-thin and had flexibility, visual transparency to a certain level.
- (2)
- When the growth time was constant, the fuel flow rate and the location of copper foil inserted into the flame had large influences on the synthesis. As the fuel flow rate increased and the copper foil moved upwards in the flame, the area of the carbon film increased, and the content of impurities reduced. In these experiments, the growth of the carbon film was better when the ethanol flow rate reached 2 mL/min and the copper foil was inserted into the top of the flame.
- (3)
- When the fuel flow rate and the copper foil location were optimal, the local growth of amorphous carbon film had occurred in such a short time of 10 s. The content of sp2 bonds was higher, the structure of entire carbon film was loose, and the surface was not smooth. When the growth time was 30 s, the content of sp3 bonds in the carbon film was maximum, the disorder degree increased, and RMS roughness was smallest. With the increase of the growth time, the carbon film gradually covered the entire surface of the copper foil, the growth of the carbon film almost stopped. The degree of order increased due to the high temperature in the flame for a longer time, and the degree of graphitization rose. When the growth time increased to 10 min, there were some obvious impurities on the carbon film surface, and the roughness of the carbon film was largest.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wal, R.L.V.; Hall, L.J. Carbon nanotube synthesis upon stainless steel meshes. Carbon 2003, 41, 659–672. [Google Scholar]
- Merchan, W.M.; Saveliev, A.V.; Kennedy, L.; Jimenez, W.C. Combustion synthesis of carbon nanotubes and related nanostructures. Prog. Energy Combust. Sci. 2010, 36, 696–727. [Google Scholar] [CrossRef]
- Szymanski, L.; Kolacinski, Z.; Wiak, S.; Raniszewski, G.; Pietrzak, L. Synthesis of carbon nanotubes in thermal plasma reactor at atmospheric pressure. Nanomaterials 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gupta, A.P.; Yeo, S.J.; Jung, J.; Paik, S.H.; Mativenga, M.; Kim, S.H.; Shin, J.H.; Ahn, J.S.; Ryu, J. Carbon nanotube field emitters synthesized on metal Alloy substrate by PECVD for customized compact field emission devices to be used in X-ray source applications. Nanomaterials 2018, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Memon, N.K.; Kear, B.H.; Tse, S.D. Transition between graphene-film and carbon-nanotube growth on Nickel alloys in open-atmosphere flame synthesis. Chem. Phys. Lett. 2013, 570, 90–94. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, G.; Wang, H.; Wang, B.; Chen, Z.; Zhang, Y.; Wang, B.; Shi, X.; Xie, X.; Jin, Z.; et al. Synthesis of large-area graphene on molybdenum foils by chemical vapor deposition. Carbon 2012, 50, 5226–5231. [Google Scholar] [CrossRef]
- Huang, L.; Chang, Q.H.; Guo, G.L.; Liu, Y.; Xie, Y.Q.; Wang, T.; Ling, B.; Yang, H.F. Synthesis of high-quality graphene films on nickel foils by rapid thermal chemical vapor deposition. Carbon 2012, 50, 551–556. [Google Scholar] [CrossRef]
- Paulo, S.; Palomares, E.; Ferrero, E.M. Graphene and carbon quantum Dot-based materials in photovoltaic devices: From synthesis to applications. Nanomaterials 2016, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Nanostructured graphene: An active component in optoelectronic devices. Nanomaterials 2018, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H.; Zakhidov, A.A.; Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Y.; Bol, A.A.; Jenkins, K.A.; Xia, F.; Farmer, D.B.; Zhu, Y.; Avouris, P. High-frequency, scaled graphene transistors on diamond-like carbon. Nature 2011, 472, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, H.; Lu, Y.; Wei, S.; Wujcik, E.K.; Guo, Z. Multifunctional carbon nanostructures for advanced energy storage applications. Nanomaterials 2015, 5, 755–777. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.E.; Kim, S.Y.; Lee, S.H. Effect of continuous multi-walled carbon nanotubes on thermal and mechanical properties of flexible composite film. Nanomaterials 2016, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Zhai, Z.; Shen, H.; Chen, J.; Jiang, Y.; Tang, Q. Investigation of substrate temperature and cooling method on the properties of amorphous carbon films by hot-filament CVD with acetylene. Carbon 2017, 117, 322–330. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R. 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Coşkun, O.D.; Zerrin, T. Optical, structural and bonding properties of diamond-like amorphous carbon films deposited by DC magnetron sputtering. Diam. Relat. Mater. 2015, 56, 29–35. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, X.; Ke, P.; Wang, A. Graphite-like carbon films by high power impulse magnetron sputtering. Appl. Surf. Sci. 2013, 283, 321–326. [Google Scholar] [CrossRef]
- Sagar, R.U.R.; Zhang, X.; Xiong, C.; Yu, Y. Semiconducting amorphous carbon thin films for transparent conducting electrodes. Carbon 2014, 76, 64–70. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R. Ion-beam deposition of thin films of diamondlike carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Cui, F.Z.; Li, D.J. A review of investigations on biocompatibility of diamond-like carbon and carbon nitride films. Surf. Coat. Technol. 2000, 131, 481–487. [Google Scholar] [CrossRef]
- Omer, A.M.M.; Adhikari, S.; Adhikary, S.; Uchida, H.; Umeno, M. Photovoltaic characteristics of postdeposition iodine-doped amorphous carbon films by microwave surface wave plasma chemical vapor deposition. Appl. Phys. Lett. 2005, 87, 161912–161913. [Google Scholar] [CrossRef]
- Constantinou, M.; Nikolaou, P.; Koutsokeras, L.; Avgeropoulos, A.; Moschovas, D.; Varotsis, C.; Patsalas, P.; Kelires, P.; Constantinides, G. Metal (Ag/Ti)-containing hydrogenated amorphous carbon nanocomposite films with enhanced nanoscratch resistance: Hybrid PECVD/PVD system and microstructural characteristics. Nanomaterials 2018, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Matlak, J.; Komvopoulos, K. Friction properties of amorphous carbon ultrathin films deposited by filtered cathodic vacuum arc and radio-frequency sputtering. Thin Solid Films 2015, 579, 167–173. [Google Scholar] [CrossRef]
- Li, Q.; Chou, H.; Zhong, J.; Liu, J.; Dolocan, A.; Zhang, J.; Zhou, Y.; Ruoff, R.S.; Chen, S.; Cai, W. Growth of adlayer graphene on Cu studied by carbon isotope. Nano. Lett. 2013, 13, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Lin, W.; Huang, Z.; Zhang, L.; Li, H.; Chen, X.; Cai, W.; Ruoff, R.S.; Chen, S. Controllable seeding of single crystal graphene islands from graphene oxide flakes. Carbon 2014, 79, 406–412. [Google Scholar] [CrossRef]
- Hongrae, C.; Sanseo, K.; Hyungson, K. Pulsed laser deposition of functionally gradient diamond-like carbon (DLC) films using a 355 nm picosecond laser. Acta. Mater. 2012, 60, 6237–6246. [Google Scholar]
- Santra, T.S.; Liu, C.H.; Bhattacharyya, T.K.; Patel, P.; Barik, T.K. Characterization of diamond-like nanocomposite thin films grown by plasma enhanced chemical vapor deposition. J. Appl. Phys. 2010, 107, 124320–124329. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2008, 47, 313–347. [Google Scholar] [CrossRef]
- Kammler, H.K.; Madler, L.; Pratsinis, S.E. Flame synthesis of nanoparticles. Chem. Eng. Technol. 2001, 24, 583–596. [Google Scholar] [CrossRef]
- Memon, N.K.; Tse, S.D.; Chhowalla, M.; Kear, B.H. Role of substrate, temperature, and hydrogen on the flame synthesis of graphene films. Proce. Combust. Inst. 2013, 34, 2163–2170. [Google Scholar] [CrossRef]
- Xu, F.; Liu, X.; Tse, S.D. Synthesis of carbon nanotubes on metal alloy substrates with voltage bias in methane inverse diffusion flames. Carbon 2006, 44, 570–577. [Google Scholar] [CrossRef]
- Goel, A.; Hebgen, P.; Sande, J.B.V.; Howard, J.B. Combustion synthesis of fullerenes and fullerenic nanostructures. Carbon 2002, 40, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Height, M.J.; Howard, J.B.; Tester, J.W.; Sande, J.B.V. Flame synthesis of single-walled carbon nanotubes. Carbon 2004, 42, 2295–2307. [Google Scholar] [CrossRef]
- Naha, S.; Sen, S.; Puri, I.K. Flame synthesis of superhydrophobic amorphous carbon surfaces. Carbon 2007, 45, 1696–1716. [Google Scholar] [CrossRef]
- Memon, N.K.; Tse, S.D.; Sharab, J.F.A.; Yamaguchi, H.; Goncalves, A.M.B.; Kear, B.H.; Jaluria, Y.; Andrei, E.Y.; Chhowalla, M. Flame synthesis of graphene films in open environments. Carbon 2011, 49, 5064–5070. [Google Scholar] [CrossRef] [Green Version]
- Sidebotham, G.W.; Glassman, I. Flame temperature, fuel structure, and fuel concentration effects on soot formation in inverse diffusion flame. Combust. Flame 1992, 90, 269–283. [Google Scholar] [CrossRef]
- Sharma, S.; Kalita, G.; Hirano, R.; Shinde, S.M.; Papon, R.; Ohtani, H.; Tanemura, M. Synthesis of graphene crystals from solid waste plastic by chemical vapor deposition. Carbon 2014, 72, 66–73. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Wang, K.; Wei, J.; Gui, X.; Li, X.; Li, C.; Fan, L.; Sun, P.; Wu, D. Ethanol flame synthesis of highly transparent carbon thin films. Carbon 2011, 49, 237–241. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Kleinsorge, B.; Morrison, N.A.; Hart, A. Stress reduction and bond stability during thermal annealing of tetrahedral amorphous carbon. J. Appl. Phys. 1999, 85, 7191–7197. [Google Scholar] [CrossRef]
- Hellgren, N.; Macak, K.; Broitman, E.; Johansson, M.P.; Hultman, L.; Sundgren, J.E. Influence of plasma parameters on the growth and properties of magnetron sputtered CNX thin films. J. Appl. Phys. 2000, 88, 524–532. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Effects of butanol isomers additions on soot nanostructure and reactivity in normal and inverse diffusion flames. Fuel 2017, 205, 109–129. [Google Scholar] [CrossRef]
- Huang, X.J.; Ryu, S.W.; Im, H.S.; Choi, Y.K. Wet chemical needlelike assemblies of single-walled carbon nanotubes on a silicon surface. Langmuir 2007, 23, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.; Gibson, C.T.; Constantopoulos, K.; Shapter, J.G.; Ellis, A.V. Functionalization of vertically aligned carbon nanotubes with polystyrene via surface initiated reversible addition fragmentation chain transfer polymerization. Appl. Surf. Sci. 2012, 258, 2836–2843. [Google Scholar] [CrossRef]








| Experimental Conditions | Ethanol Flow Rate (mL/min) | Gas Flow Rate (L/min) | Height (mm) | Growth Time (min) | ||
|---|---|---|---|---|---|---|
| Air | Carrier N2 | Protection N2 | ||||
| E1-1 | 1.0 | 0.35 | 1.54 | 18 | 3.0 | 5 |
| E1-2 | 6.0 | |||||
| E1-3 | 10.0 | |||||
| E1-4 | 12.0 | |||||
| E2-1 | 1.5 | 0.35 | 2.31 | 18 | 3.5 | 5 |
| E2-2 | 6.5 | |||||
| E2-3 | 11.0 | |||||
| E2-4 | 13.0 | |||||
| E3-1 | 2.0 | 0.35 | 3.08 | 18 | 4.0 | 5 |
| E3-2 | 7.0 | |||||
| E3-3 | 12.0 | |||||
| E3-4 | 14.0 | |||||
| E3-5 | 14.0 | 1/6 | ||||
| E3-6 | 14.0 | 1/2 | ||||
| E3-7 | 14.0 | 2 | ||||
| E3-8 | 14.0 | 10 | ||||
| Growth Time | Peak Position (cm−1) | Ratio of Peak Areas | FWHM (cm−1) | |
|---|---|---|---|---|
| G | D | ID/IG | G | |
| 10 s | 1601 | 1361 | 2.54 | 88.6 |
| 30 s | 1593 | 1356 | 1.98 | 109.6 |
| 2 min | 1598 | 1351 | 2.16 | 98.9 |
| 5 min | 1603 | 1351 | 4.56 | 74.4 |
| 10 min | 1606 | 1359 | 6.00 | 68.6 |
| Growth Time | C (at.%) | N (at.%) | O (at.%) |
|---|---|---|---|
| 30 s | 82.88 | 1.30 | 15.83 |
| 2 min | 61.08 | 1.18 | 37.74 |
| 5 min | 54.25 | 1.37 | 44.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, F.; Liu, G.; Liu, D.; Li, Q.; Kan, E. Synthesis of Amorphous Carbon Film in Ethanol Inverse Diffusion Flames. Nanomaterials 2018, 8, 656. https://doi.org/10.3390/nano8090656
Zhu J, Li F, Liu G, Liu D, Li Q, Kan E. Synthesis of Amorphous Carbon Film in Ethanol Inverse Diffusion Flames. Nanomaterials. 2018; 8(9):656. https://doi.org/10.3390/nano8090656
Chicago/Turabian StyleZhu, Jie, Fang Li, Guannan Liu, Dong Liu, Qiongyu Li, and Erjun Kan. 2018. "Synthesis of Amorphous Carbon Film in Ethanol Inverse Diffusion Flames" Nanomaterials 8, no. 9: 656. https://doi.org/10.3390/nano8090656





