Zinc Oxide Nanoparticles from Waste Zn-C Battery via Thermal Route: Characterization and Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization Methods
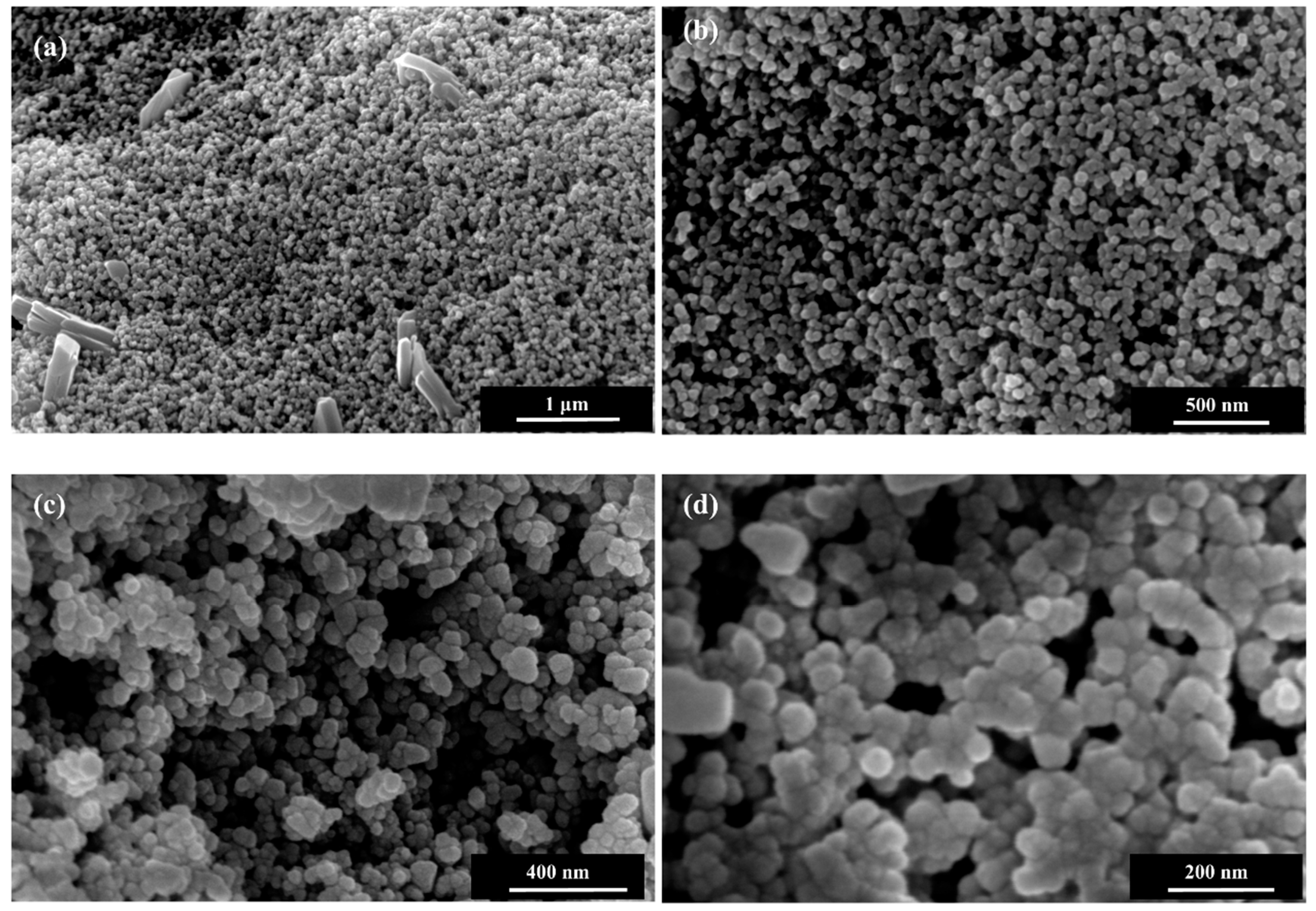
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayaraman, V.K.; Álvarez, A.M.; Amador, M.d.l.L.O. A simple and cost-effective zinc oxide thin film sensor for propane gas detection. Mater. Lett. 2015, 157, 169–171. [Google Scholar] [CrossRef]
- İpeksaç, T.; Kaya, F.; Kaya, C. Hydrothermal synthesis of zinc oxide (ZnO) nanotubes and its electrophoretic deposition on nickel filter. Mater. Lett. 2013, 100, 11–14. [Google Scholar] [CrossRef]
- Khan, W.; Khan, F.; Ajmal, H.M.S.; Huda, N.U.; Kim, J.H.; Kim, S.-D. Evolution of structural and optical properties of ZnO nanorods grown on vacuum annealed seed crystallites. Nanomaterials 2018, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Mueller, F.; Fiedler, M.; Krueger, S.; Kloepsch, R.; Baither, D.; Winter, M.; Paillard, E.; Passerini, S. Transition-metal-doped zinc oxide nanoparticles as a new lithium-ion anode material. Chem. Mater. 2013, 25, 4977–4985. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Effect of microwave radiation power on the size of aggregates of ZnO nps prepared using microwave solvothermal synthesis. Nanomaterials 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Giuli, G.; Trapananti, A.; Mueller, F.; Bresser, D.; d’Acapito, F.; Passerini, S. Insights into the effect of iron and cobalt doping on the structure of nanosized ZnO. Inorg. Chem. 2015, 54, 9393–9400. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, C.; Pearton, S.J. Basic Properties and Applications of ZnO, Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications; Elsevier Science: Oxford, UK, 2006. [Google Scholar]
- Bhatte, K.D.; Sawant, D.N.; Watile, R.A.; Bhanage, B.M. A rapid, one step microwave assisted synthesis of nanosize zinc oxide. Mater. Lett. 2012, 69, 66–68. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Huang, K.-L.; Lee, J.-F. Preparation and morphology studies of nano zinc oxide obtained using native and modified chitosans. Materials 2013, 6, 4198–4212. [Google Scholar] [CrossRef] [PubMed]
- Zinc Price Surges on Supply Shortage, Renewable Development Keeps Copper on Steady Growth. Available online: http://www.Abc.Net.Au/news/2016-08-05/zinc-copper-price-surge/7690040 (accessed on 8 August 2018).
- Kiddee, P.; Naidu, R.; Wong, M.H. Electronic waste management approaches: An overview. Waste Manag. 2013, 33, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- World Batteries-Demand and Sales Forecasts, Market Share, Market Size, Market Leaders. Available online: http://www.Freedoniagroup.Com/world-batteries.html (accessed on 10 July 2018).
- Analysis of Battery Consumption, Recycling and Disposal in Australia. Warnken Industrial and Social Ecology Pty Ltd., 2010. Available online: http://www.batteryrecycling.org.au/wp-content/uploads/2011/06/Battery-consumption-recycling-and-disposal-in-Australia_Executive-Summary.pdf (accessed on 2 July 2018).
- Ebin, B.; Petranikova, M.; Steenari, B.-M.; Ekberg, C. Production of zinc and manganese oxide particles by pyrolysis of alkaline and Zn–C battery waste. Waste Manag. 2016, 51, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Charef, S.A.; Affoune, A.; Caballero, A.; Cruz-Yusta, M.; Morales, J. Simultaneous recovery of zn and mn from used batteries in acidic and alkaline mediums: A comparative study. Waste Manag. 2017, 68, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Sharma, A.L.; Mohanta, G.C.; Kumar, P.; Kim, K.-H. A facile chemical route for recovery of high quality zinc oxide nanoparticles from spent alkaline batteries. Waste Manag. 2016, 51, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sobianowska-Turek, A.; Szczepaniak, W.; Maciejewski, P.; Gawlik-Kobylińska, M. Recovery of zinc and manganese, and other metals (Fe, Cu, Ni, Co, Cd, Cr, Na, K) from Zn-MnO2 and Zn-C waste batteries: Hydroxyl and carbonate co-precipitation from solution after reducing acidic leaching with use of oxalic acid. J. Power Sources 2016, 325, 220–228. [Google Scholar] [CrossRef]
- Cayumil, R.; Khanna, R.; Rajarao, R.; Mukherjee, P.S.; Sahajwalla, V. Concentration of precious metals during their recovery from electronic waste. Waste Manag. 2016, 57, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.R.; Aravinda, L.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Activated carbon derived from non-metallic printed circuit board waste for supercapacitor application. Electrochim. Acta 2016, 211, 488–498. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Hassan, K.; Behera, P.R.; Sahajwalla, V. Thermal nanosizing: Novel route to synthesize manganese oxide and zinc oxide nanoparticles simultaneously from spent Zn–C battery. J. Clean. Prod. 2018, 196, 478–488. [Google Scholar] [CrossRef]
- Rasines, I.; de Setién, J.M. Thermal analysis of β-Co2(OH)3Cl and Zn5(OH)5Cl2·H2O. Thermochim. Acta 1980, 37, 239–246. [Google Scholar] [CrossRef]
- Mohanan, A.A.; Parthiban, R.; Ramakrishnan, N. Alignment nature of ZnO nanowires grown on polished and nanoscale etched lithium niobate surface through self-seeding thermal evaporation method. Materials Res. Bull. 2015, 68, 35–41. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829. [Google Scholar] [CrossRef]
- Yu, D.; Trad, T.; McLeskey, J.T.; Craciun, V.; Taylor, C.R. ZnO nanowires synthesized by vapor phase transport deposition on transparent oxide substrates. Nanoscale Res. Lett. 2010, 5, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, B.; Pan, Z.; Gu, M.; Punnoose, A. Synthesis of ZnO nanoparticles with controlled shapes, sizes, aggregations, and surface complex compounds for tuning or switching the photoluminescence. Cryst. Growth Des. 2015, 15, 3144–3149. [Google Scholar] [CrossRef]
- Das, J.; Pradhan, S.; Sahu, D.; Mishra, D.; Sarangi, S.; Nayak, B.; Verma, S.; Roul, B. Micro-raman and xps studies of pure ZnO ceramics. Phys. B Condens. Matter 2010, 405, 2492–2497. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. Xps and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Herrera-Rivera, R.; Olvera, M.; Maldonado, A. Synthesis of ZnO nanopowders by the homogeneous precipitation method: Use of taguchi’s method for analyzing the effect of different variables. J. Nanomater. 2017, 2017, 4595384. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Hussein, M.Z.B.; Taufiq-Yap, Y.H. Synthesis and characterization of ZnO nanostructures using palm olein as biotemplate. Chem. Cent. J. 2013, 7, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pudukudy, M.; Hetieqa, A.; Yaakob, Z. Synthesis, characterization and photocatalytic activity of annealing dependent quasi spherical and capsule like ZnO nanostructures. Appl. Surf. Sci. 2014, 319, 221–229. [Google Scholar] [CrossRef]
- Singh, R.P.P.; Hudiara, I.; Panday, S.; Rana, S.B. The effect of Co doping on the structural, optical, and magnetic properties of Fe-doped ZnO nanoparticles. J. Supercond. Novel Magn. 2016, 29, 819–827. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced uv photodetector and room temperature no sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [PubMed]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, Y.; Liu, Y.; Zhang, G.; Zhang, X. Photoluminescence spectra of nano-structured ZnO thin films. J. Lumin. 2006, 119–120, 233–236. [Google Scholar] [CrossRef]
- Nagaraju, G.; Nagabhushana, H.; Suresh, D.; Anupama, C.; Raghu, G.; Sharma, S. Vitis labruska skin extract assisted green synthesis of ZnO super structures for multifunctional applications. Ceram. Int. 2017, 43, 11656–11667. [Google Scholar]








| Assigned Miller Indices (hkl) | Calculated d Spacing (nm) | Observed d Spacing (nm) | Particle Size (nm) |
|---|---|---|---|
| 100 | 0.285 | 0.281 | 27.93 |
| 002 | 0.262 | 0.260 | 28.12 |
| 101 | 0.249 | 0.248 | 28.06 |
| 110 | 0.163 | 0.162 | 26.43 |
| Name | Start BE | Peak BE | End BE | FWMH eV | Atomic % |
|---|---|---|---|---|---|
| O1sA | 534.79 | 530.36 | 526.69 | 1.4 | 26.53 |
| O1sB | 534.79 | 531.56 | 526.69 | 1.4 | 13.63 |
| O1sC | 534.79 | 532.68 | 526.69 | 1.39 | 3.61 |
| C1sA | 294.99 | 284.80 | 281.39 | 1.49 | 6.84 |
| C1sB | 294.99 | 286.47 | 281.39 | 1.49 | 2.12 |
| C1sC | 294.99 | 287.80 | 281.39 | 1.49 | 0.62 |
| C1sD | 294.99 | 289.30 | 281.39 | 1.49 | 1.58 |
| K2p3 | 294.99 | 293.32 | 281.39 | 1.35 | 0.31 |
| Ca2p3A | 355.09 | 347.36 | 343.99 | 1.94 | 0.20 |
| Cl2p3A | 203.09 | 198.83 | 195.79 | 1.47 | 5.49 |
| N1sA | 404.79 | 400.90 | 396.29 | 1.85 | 1.79 |
| Na1s | 1076.69 | 1072.26 | 1068.09 | 1.74 | 3.73 |
| Si2p | 104.49 | 101.84 | 99.09 | 1.39 | 3.18 |
| Zn2p3 | 1026.49 | 1021.87 | 1017.49 | 1.9 | 29.96 |
| Name | Results |
|---|---|
| BET Surface area | 9.2629 m2/g |
| Total pore volume of pores at p/p0 = 0.95 | 0.0536 cm3/g |
| Adsorption average pore diameter (4 V/A by BET) | 23.17 nm |
| BJH Adsorption average pore width (4 V/A) | 4.93 nm |
| BJH Desorption average pore width (4 V/A) | 4.82 nm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farzana, R.; Rajarao, R.; Behera, P.R.; Hassan, K.; Sahajwalla, V. Zinc Oxide Nanoparticles from Waste Zn-C Battery via Thermal Route: Characterization and Properties. Nanomaterials 2018, 8, 717. https://doi.org/10.3390/nano8090717
Farzana R, Rajarao R, Behera PR, Hassan K, Sahajwalla V. Zinc Oxide Nanoparticles from Waste Zn-C Battery via Thermal Route: Characterization and Properties. Nanomaterials. 2018; 8(9):717. https://doi.org/10.3390/nano8090717
Chicago/Turabian StyleFarzana, Rifat, Ravindra Rajarao, Pravas Ranjan Behera, Kamrul Hassan, and Veena Sahajwalla. 2018. "Zinc Oxide Nanoparticles from Waste Zn-C Battery via Thermal Route: Characterization and Properties" Nanomaterials 8, no. 9: 717. https://doi.org/10.3390/nano8090717





