Aggregation-Induced Emission of Tetraphenylethene-Conjugated Phenanthrene Derivatives and Their Bio-Imaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Preparation of pTPEP and pTPEP Derivatives
2.4. General Procedure for the Suzuki Coupling for the Preparation of pTPEP and mTPEP
2.5. Synthesis of TPEP Derivatives-Loaded Silica Nanoparticles (mTPEP-SiO2 and pTPEP-SiO2)
2.6. Entrapment Efficiency
2.7. Photostability of Nanoparticles
2.8. Cell Culture and Bio-Imaging Experiment
3. Results and Discussion
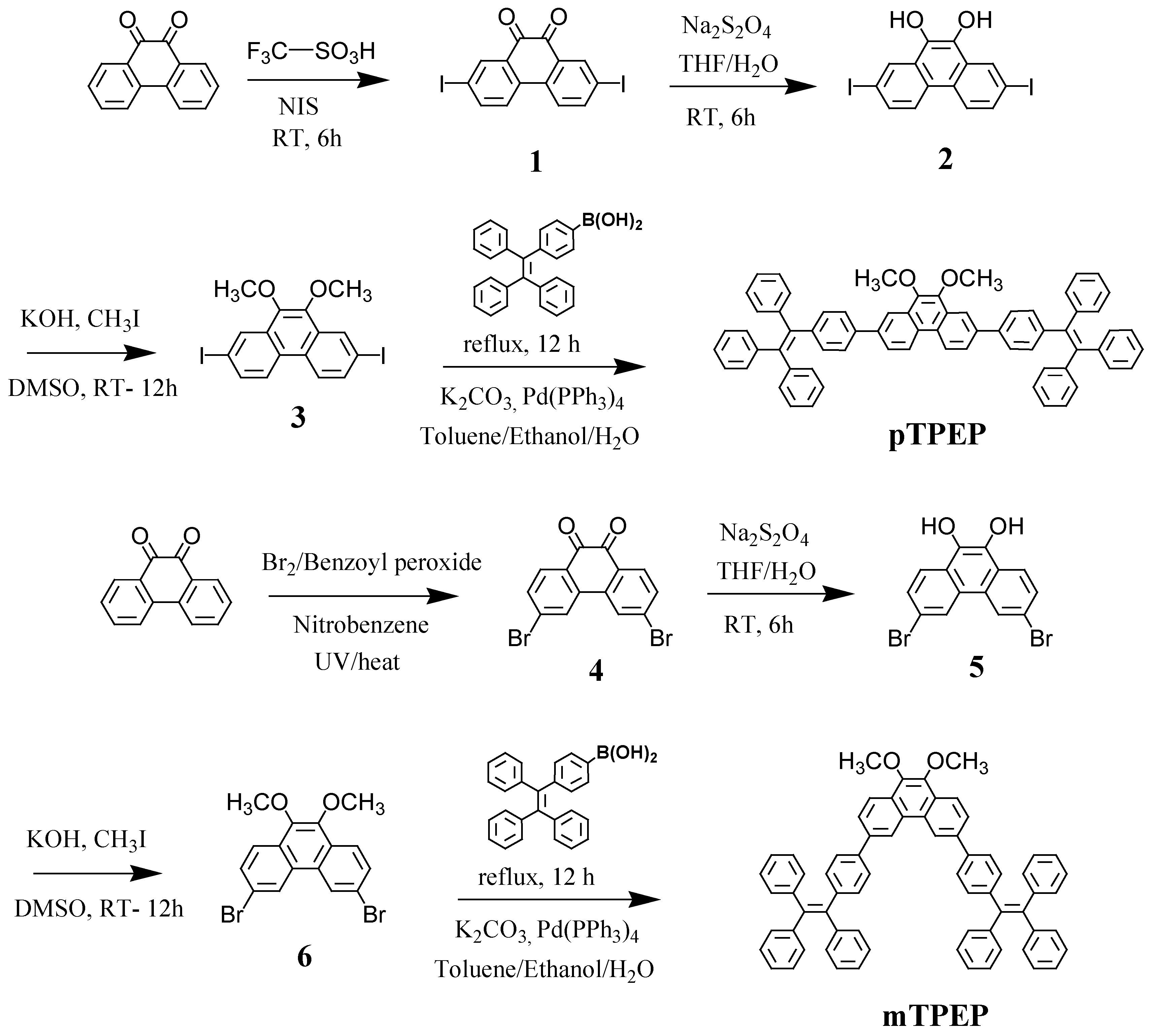
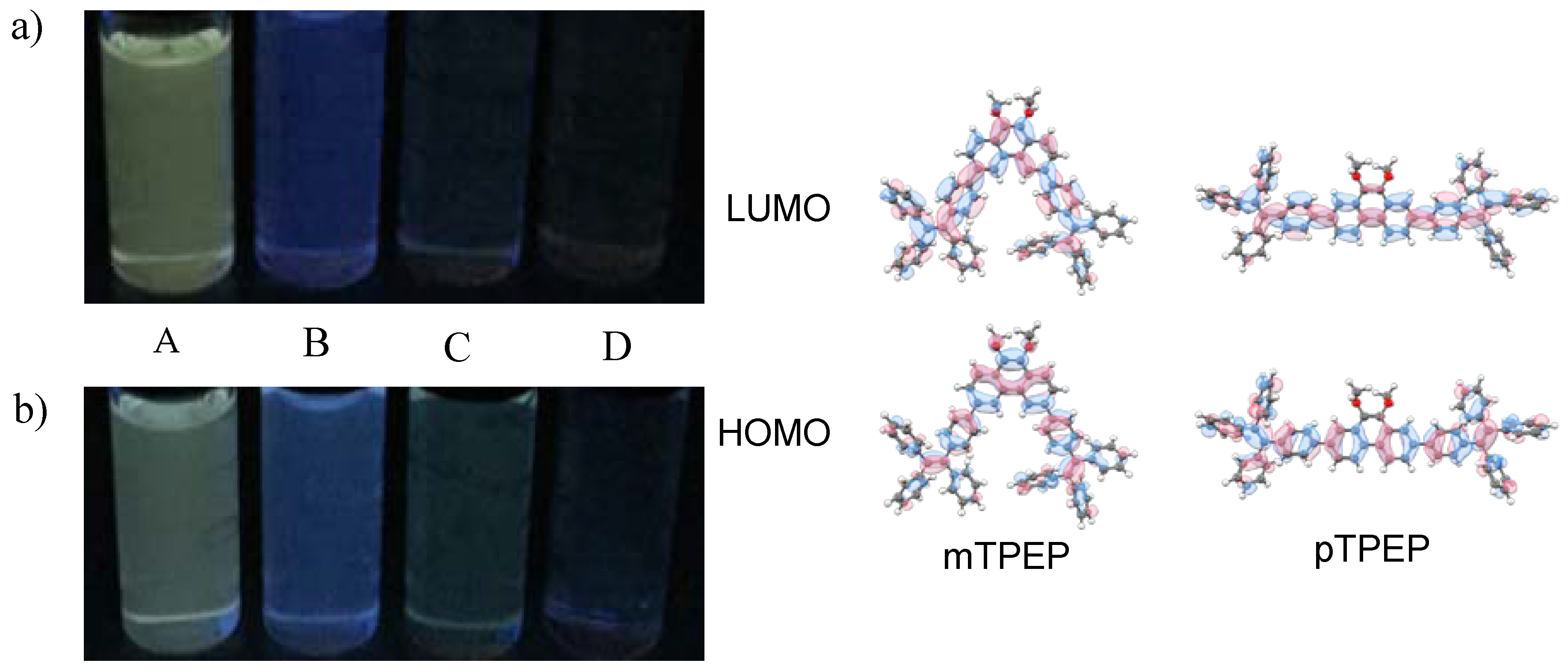
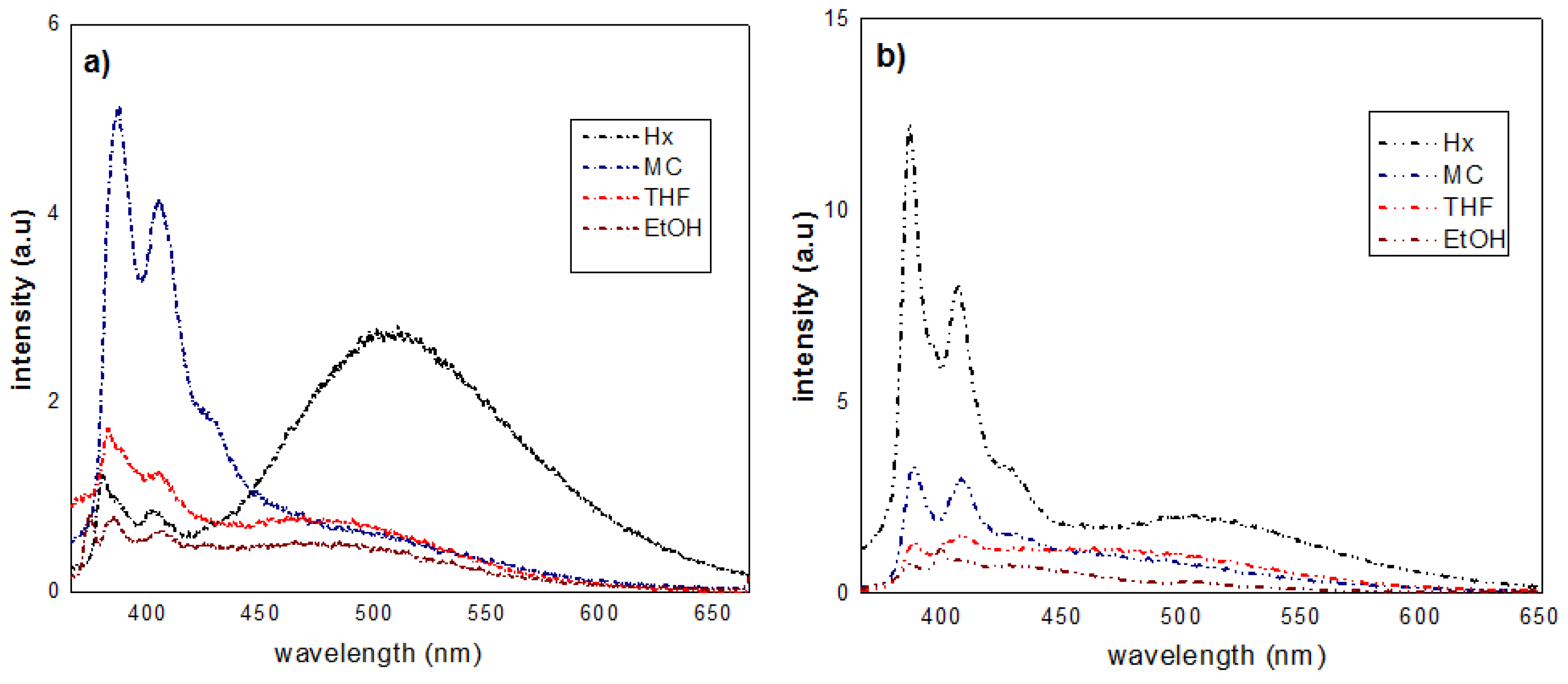
3.1. Synthesis and Optical Properties
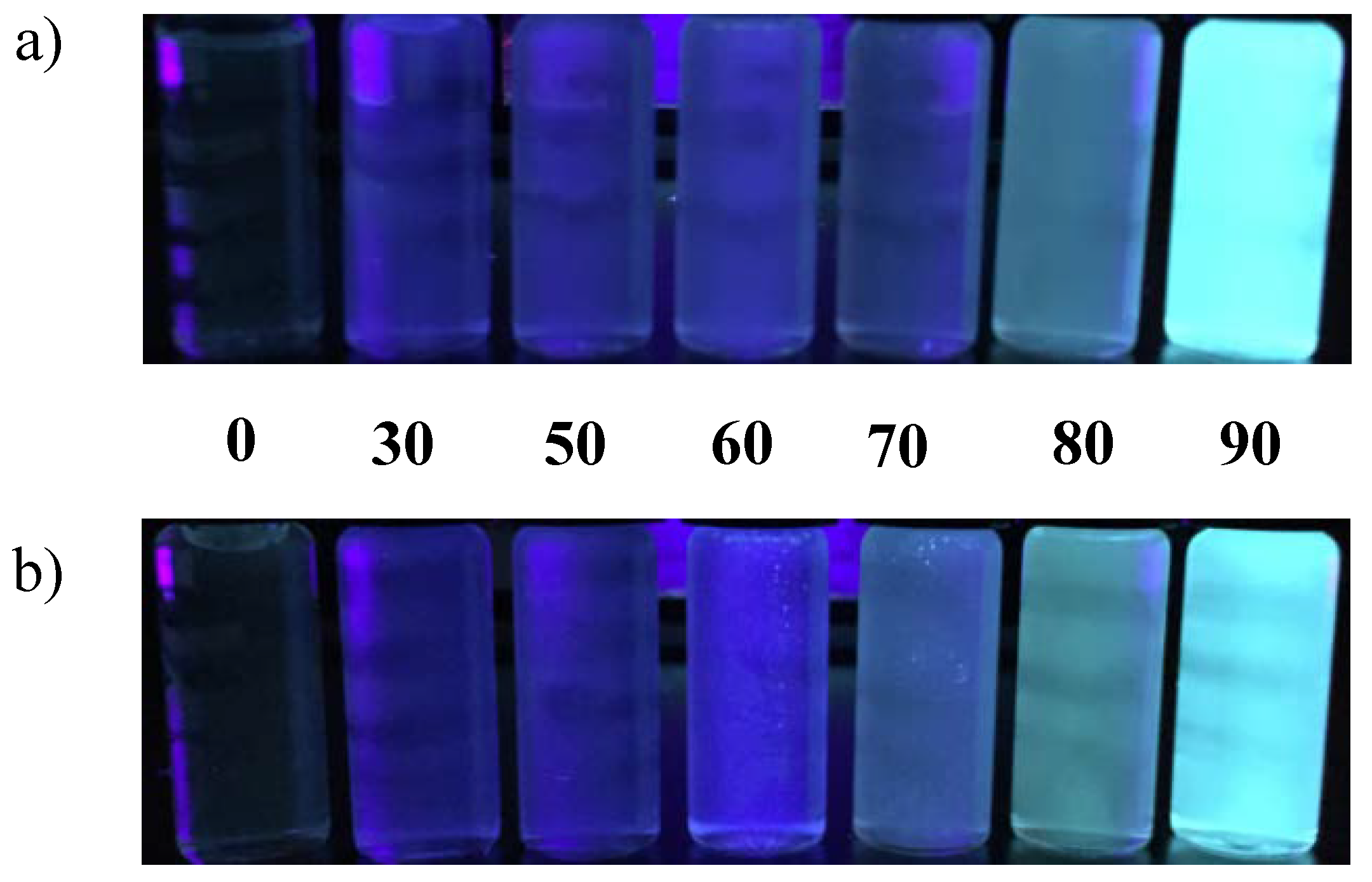
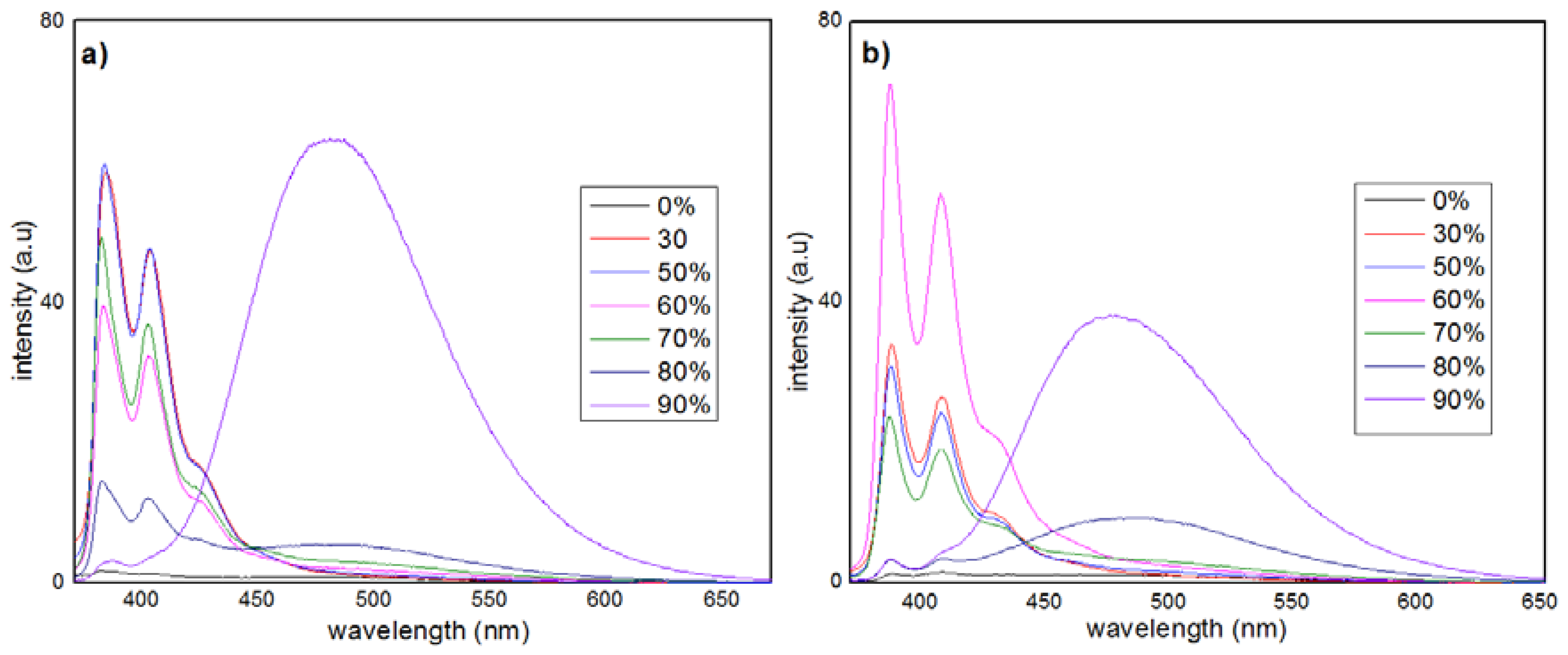
3.2. AIE Properties
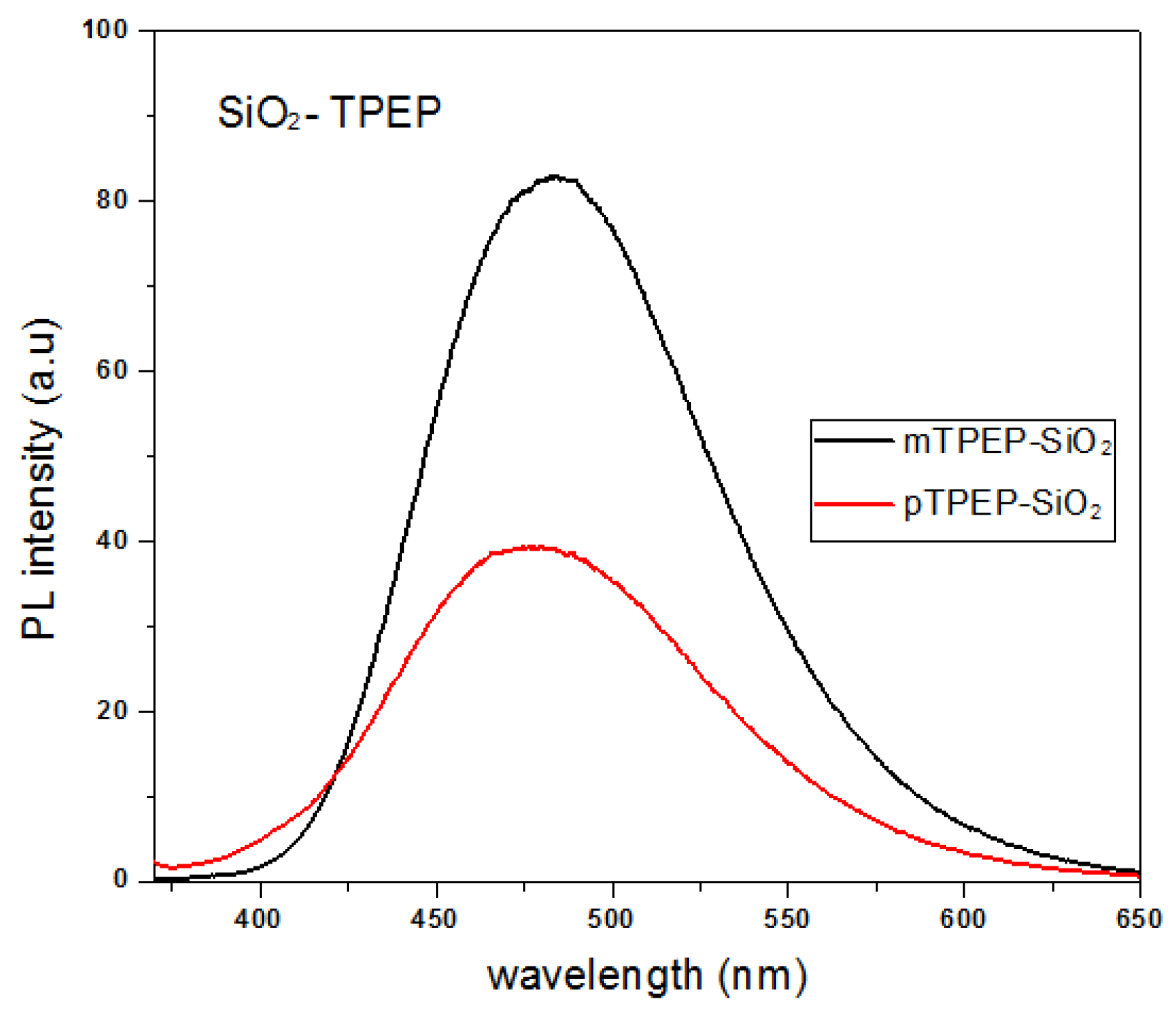
3.3. Synthesis and Characterization of TPEP-SiO2 Nanoparticles
3.3.1. Size Determination
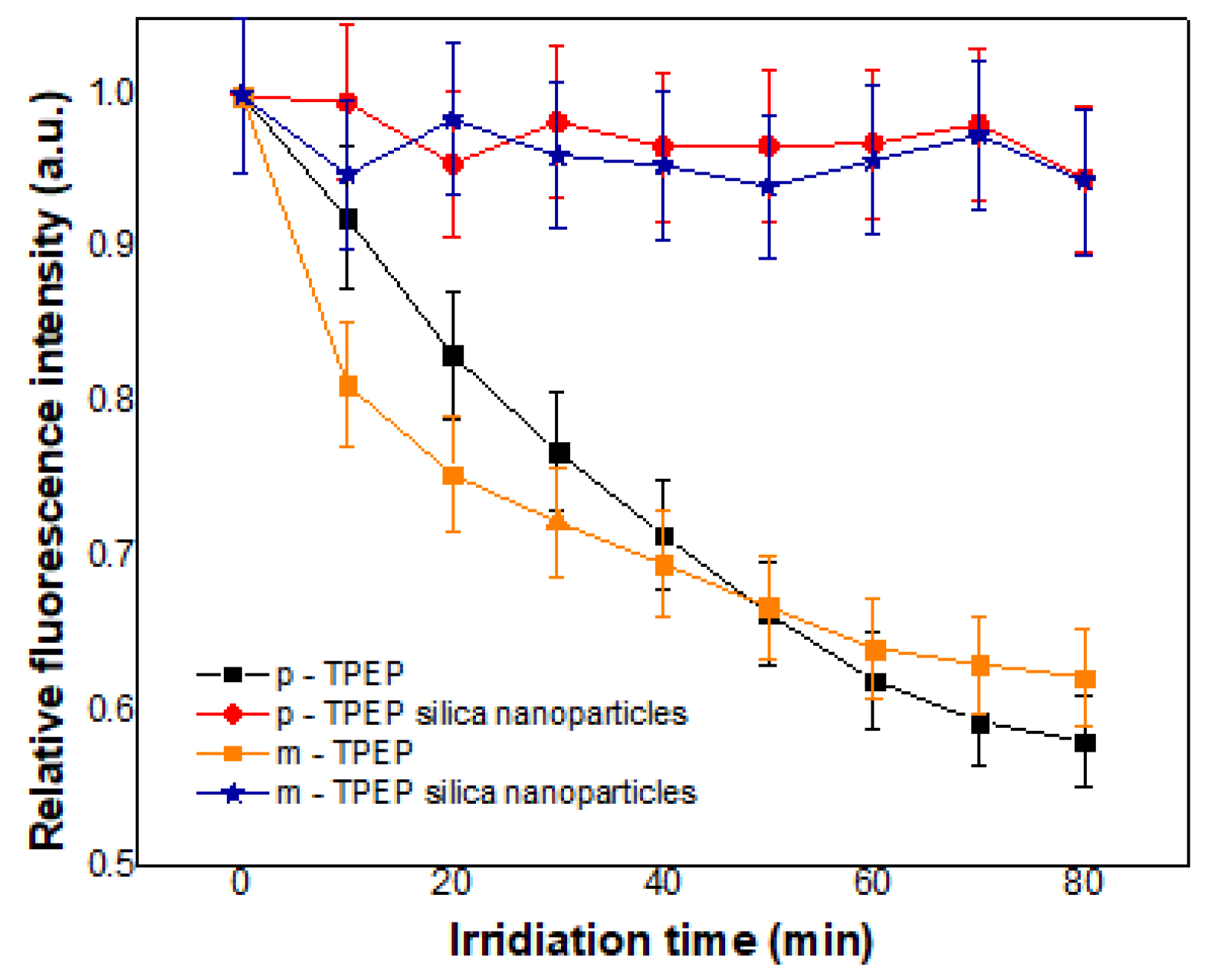
3.3.2. Photobleaching
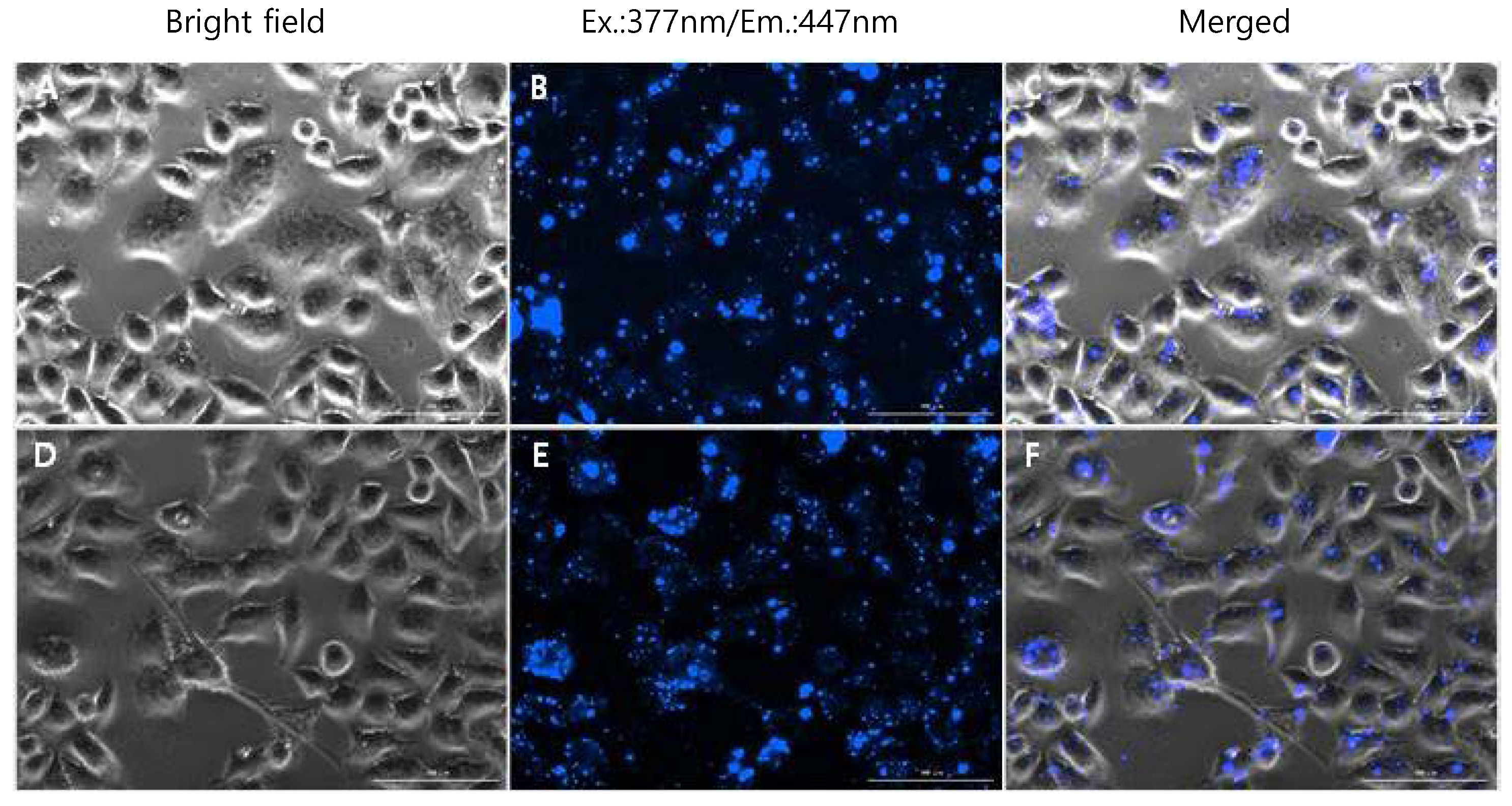
3.4. TPEP-SiO2 for Cell Imaging
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.H.; Hoa, T.H.; Temmy, P.V.; Cho, S.; Kim, H.J. Multi-color fluorescence of pNIPAM-Based nanogels modulated by dual stimuli-responsive FRET processes. Dyes Pigments 2016, 145, 216–221. [Google Scholar] [CrossRef]
- Jadhav, T.; Choi, J.M.; Lee, J.Y.; Dhokale, B.; Misra, R. Non-doped blue organic light emitting devices based on tetraphenylethylene-π-imidazole derivatives. Org. Electron. 2016, 37, 448–452. [Google Scholar] [CrossRef]
- Tang, X.; Bai, Q.; Peng, Q.; Gao, Y.; Li, J.; Liu, Y.; Yao, L.; Lu, P.; Yang, B.; Ma, Y. Efficient deep blue electroluminescence with an external quantum efficiency of 6.8% and CIEy < 0.08 based on a phenanthroimidazole-sulfone hybrid donor-acceptor molecule. Chem. Mater. 2015, 27, 7050–7057. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Leok Chan, K.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Xie, Y.; Wang, C.; Li, J.-R.; Li, Q.; Li, Z. From ACQ to AIE: The suppression of the strong π–π interaction of naphthalene diimide derivatives through the adjustment of their flexible chains. Chem. Commun. 2016, 52, 11496–11499. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, M.; Zhai, D.; Er, J.C.; Chang, Y.-T. Combinatorial strategies in fluorescent probe development. Chem. Rev. 2012, 112, 4391–4420. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Faisal, M.; Hong, Y.; Liu, J.; Yu, Y.; Lam, J.W.Y.; Qin, A.; Lu, P.; Tang, B.Z. Fabrication of fluorescent silica nanoparticles hybridized with AIE luminogens and exploration of their applications as nanobiosensors in intracellular imaging. Chem. Eur. J. 2010, 16, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, S.; Shen, X.; Mahtab, F.; Yu, Y.; Lu, P.; Lam, J.W.Y.; Kwok, H.S.; Tang, B.Z. Aggregation-induced emission, self-assembly and electroluminescence of 4,4’-bis(1,2,2-triphenylvinyl)biphenyl. Chem. Commun. 2010, 46, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, S.; Chan, C.Y.K.; Lam, J.W.Y.; Jim, C.K.W.; Liu, P.; Chang, Z.; Kwok, H.S.; Qiu, H.; Tang, B.Z. A facile and versatile approach to efficient luminescent materials for applications in organic light-emitting diodes. Chem. Asian J. 2012, 7, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yin, S.; Liu, Y.; Chen, J.; Xu, X.; Sun, X.; Ma, D.; Zhan, X.; Peng, Q.; Shuai, Z.; et al. Structures, electronic states, photoluminescence, and carrier transport properties of 1,1-disubstituted 2,3,4,5-tetraphenylsiloles. J. Am. Chem. Soc. 2005, 127, 6335–6346. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lam, J.W.Y.; Qin, A.; Liu, J.; Li, Z.; Tang, B.Z.; Sun, J.; Kwok, H.S. Aggregation-induced emissions of tetraphenylethene derivatives and their utilities as chemical vapor sensors and in organic light-emitting diodes. Appl. Phys. Lett. 2007, 91, 011111. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Huang, J.; Li, Q.; Li, Z. Blue AIEgens: approaches to control the intramolecular conjugation and the optimized performance of OLED devices. J. Mater. Chem. C 2016, 4, 2663–2684. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhang, D.; Zhu, D.; Tang, B.Z. Fluorescent bio/chemosensors based on silole and tetraphenylethene luminogens with aggregation-induced emission feature. J. Mater. Chem. 2010, 20, 1858–1867. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Lam, J.W.Y.; Hong, Y.; Faisal, M.; Yuan, W.Z.; Tang, B.Z. Simple biosensor with high selectivity and sensitivity: Thiol-specific biomolecular probing and intracellular imaging by AIE fluorogen on a TLC plate through a Thiol-Ene click mechanism. Chem. Eur. J. 2010, 16, 8433–8438. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. Bodipy-doped silica nanoparticles with reduced dye leakage and enhanced singlet oxygen generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.E.; Lee, J.; Jang, Y.; Kim, S.-W.; An, K.; Yu, J.H.; Hyeon, T. Generalized fabrication of multifunctional nanoparticle assemblies on silica spheres. Angew. Chem. 2006, 118, 4907–4911. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jung, E.-Y.; Jin, L.Y.; Lee, M. Solution behavior of dendrimer-coated rodlike coordination polymers. Macromolecules 2008, 41, 6066–6072. [Google Scholar] [CrossRef]
- Kobin, B.; Grubert, L.; Blumstengel, S.; Henneberger, F.; Hecht, S. Vacuum-processable ladder-type oligophenylenes for organic-inorganic hybrid structures: Synthesis, optical and electrochemical properties upon increasing planarization as well as thin film growth. J. Mater. Chem. 2012, 22, 4383–4390. [Google Scholar] [CrossRef]
- Brunner, K.; van Dijken, A.; Börner, H.; Bastiaansen, J.J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W. Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: Tuning the homo level without influencing the triplet energy in small molecules. J. Am. Chem. Soc. 2004, 126, 6035–6042. [Google Scholar] [CrossRef] [PubMed]
- Speck, M.; Niethammer, D.; Senge, M.O. Isomeric porphyrin phenanthrenequinones: Synthesis, NMR spectroscopy, electrochemical properties, and in situ EPR/ENDOR studies of the o-semiquinone anion radicals. J. Chem. Soc. Perk. T. 2 2002, 455–462. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Bouchmella, K.; Picco, A.S.; Capeletti, L.B.; Gonçalves, K.A.; Santos, J.H.Z.D.; Kobarg, J.; Cardoso, M.B. Tailored silica nanoparticles surface to increase drug load and enhance bactericidal response. J. Braz. Chem. Soc. 2017, 28, 1715–1724. [Google Scholar] [CrossRef]
- Kardys, A.Y.; Bharali, D.J.; Mousa, S.A. Amino-functionalized silica nanoparticles: In vitro evaluation for targeted delivery and therapy of pancreatic cancer. J. Nanotechnol. 2013, 2013, 8. [Google Scholar] [CrossRef]
- Wu, G.; Zeng, F.; Wu, S. A water-soluble and specific BODIPY-based fluorescent probe for hypochlorite detection and cell imaging. Anal. Methods 2013, 5, 5589–5596. [Google Scholar] [CrossRef]
- Hong, X.; Wang, Z.; Yang, J.; Zheng, Q.; Zong, S.; Sheng, Y.; Zhu, D.; Tang, C.; Cui, Y. Silylated BODIPY dyes and their use in dye-encapsulated silica nanoparticles with switchable emitting wavelengths for cellular imaging. Analyst 2012, 137, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Sun, N.; Wu, Z.; Tu, J.; Yuan, L.; Tang, X.; Xie, Y.; Peng, Q.; Dong, Y.; Li, Q.; et al. Polyphenylbenzene as a platform for deep-blue OLEDs: Aggregation enhanced emission and high external quantum efficiency of 3.98%. Chem. Mater. 2015, 27, 1847–1854. [Google Scholar] [CrossRef]
- Tong, H.; Dong, Y.; Häußler, M.; Hong, Y.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Kwok, H.S.; Tang, B.Z. Molecular packing and aggregation-induced emission of 4-dicyanomethylene-2,6-distyryl-4H-pyran derivatives. Chem. Phys. Lett. 2006, 428, 326–330. [Google Scholar] [CrossRef]
- Tracy, H.J.; Mullin, J.L.; Klooster, W.T.; Martin, J.A.; Haug, J.; Wallace, S.; Rudloe, I.; Watts, K. Enhanced photoluminescence from group 14 metalloles in aggregated and solid solutions. Inorg. Chem. 2005, 44, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Chen, Z.; Zhang, Q.; Yan, Y.; Qian, S.; Cao, Y.; Tian, H. Aggregation-induced emission (AIE)-active starburst triarylamine fluorophores as potential non-doped red emitters for organic light-emitting diodes and Cl2 gas chemodosimeter. Adv. Funct. Mater. 2007, 17, 3799–3807. [Google Scholar] [CrossRef]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesner, U. Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett. 2005, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]

| Solvent | mTPEP | pTPEP | ||||
|---|---|---|---|---|---|---|
| λab (nm) | λem (nm) | ΦF | λab (nm) | λem (nm) | ΦF | |
| Hexane | 329 | 377/401 | 0.016 | 308/345 | 384/406 | 0.043 |
| THF | 333 | 386/403 | 0.005 | 312/355 | 386/408 | 0.005 |
| Dichloromethane | 331 | 385/402 | 0.005 | 310/346 | 389/407 | 0.006 |
| Ethanol | 336 | 380/404 | 0.005 | nd | 382/398 | nd |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuong Mai, D.; Lee, J.; Min, I.; Vales, T.P.; Choi, K.-H.; Park, B.J.; Cho, S.; Kim, H.-J. Aggregation-Induced Emission of Tetraphenylethene-Conjugated Phenanthrene Derivatives and Their Bio-Imaging Applications. Nanomaterials 2018, 8, 728. https://doi.org/10.3390/nano8090728
Khuong Mai D, Lee J, Min I, Vales TP, Choi K-H, Park BJ, Cho S, Kim H-J. Aggregation-Induced Emission of Tetraphenylethene-Conjugated Phenanthrene Derivatives and Their Bio-Imaging Applications. Nanomaterials. 2018; 8(9):728. https://doi.org/10.3390/nano8090728
Chicago/Turabian StyleKhuong Mai, Duy, Joomin Lee, Ilgi Min, Temmy Pegarro Vales, Kyong-Hoon Choi, Bong Joo Park, Sung Cho, and Ho-Joong Kim. 2018. "Aggregation-Induced Emission of Tetraphenylethene-Conjugated Phenanthrene Derivatives and Their Bio-Imaging Applications" Nanomaterials 8, no. 9: 728. https://doi.org/10.3390/nano8090728
APA StyleKhuong Mai, D., Lee, J., Min, I., Vales, T. P., Choi, K.-H., Park, B. J., Cho, S., & Kim, H.-J. (2018). Aggregation-Induced Emission of Tetraphenylethene-Conjugated Phenanthrene Derivatives and Their Bio-Imaging Applications. Nanomaterials, 8(9), 728. https://doi.org/10.3390/nano8090728





