The Enhanced Lithium-Storage Performance for MnO Nanoparticles Anchored on Electrospun Nitrogen-Doped Carbon Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis Procedure
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
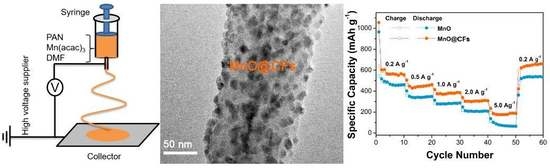
3.1. Structure and Morphology Characterization
3.2. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.L.; Shen, S.Y.; Peng, X.X.; Wu, L.N.; Wang, Q.; Shen, C.H.; Tu, T.T.; Huang, L.; Li, J.T.; Sun, S.G. New insights into the structure changes and interface properties of Li3VO4 anode for lithium-ion batteries during the initial cycle by in-situ techniques. ACS Appl. Mater. Interfaces 2016, 8, 23739–23745. [Google Scholar] [PubMed]
- Xia, Y.; Xiao, Z.; Dou, X.; Huang, H.; Lu, X.; Yan, R.; Gan, Y.; Zhu, W.; Tu, J.; Zhang, W.; et al. Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 2013, 7, 7083–7092. [Google Scholar] [PubMed]
- Hou, C.; Tai, Z.; Zhao, L.; Zhai, Y.; Hou, Y.; Fan, Y.; Dang, F.; Wang, J.; Liu, H. High performance MnO@C microcages with a hierarchical structure and tunable carbon shell for efficient and durable lithium storage. J. Mater. Chem. A 2018, 6, 9723–9736. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar]
- Guoyin, Z.; Lei, W.; Huinan, L.; Liaobo, M.; Peiyang, Z.; Yi, H.; Tao, C.; Renpeng, C.; Yanrong, W.; Zuoxiu, T.; et al. Walnut-like multicore–shell MnO encapsulated nitrogen-rich carbon nanocapsules as anode material for long-cycling and soft-packed lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1800003. [Google Scholar]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of conformal nanoscale MnO on graphene as a high-capacity and long-life anode material for lithium ion batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Yun, Z.; Penghui, C.; Xu, G.; Bo, W.; Heng, L.; Hao, W.; Huakun, L.; Shixue, D. Nitrogen-doped graphene ribbon assembled core–sheath MnO@graphene scrolls as hierarchically ordered 3D porous electrodes for fast and durable lithium storage. Adv. Funct. Mater. 2016, 26, 7754–7765. [Google Scholar]
- Ma, Z.Y.; Cao, H.L.; Zhou, X.F.; Deng, W.; Liu, Z.P. Hierarchical porous MnO/graphene composite aerogel as high-performance anode material for lithium ion batteries. RSC Adv. 2017, 7, 15857–15863. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Ma, L.; Zheng, M.; Lin, Z.; Zhao, B.; Wen, Z.; Hu, Z.; Pu, L.; Shi, Y. MnO nanoparticles anchored on graphene nanosheets via in situ carbothermal reduction as high-performance anode materials for lithium-ion batteries. Mater. Lett. 2012, 84, 9–12. [Google Scholar]
- Zhang, G.; Wu, H.B.; Hoster, H.E.; Lou, X.W. Strongly coupled carbon nanofiber-metal oxide coaxial nanocables with enhanced lithium storage properties. Energ. Environ. Sci. 2014, 7, 302–305. [Google Scholar]
- Liu, D.-S.; Liu, D.-H.; Hou, B.-H.; Wang, Y.-Y.; Guo, J.-Z.; Ning, Q.-L.; Wu, X.-L. 1D porous MnO@N-doped carbon nanotubes with improved Li-storage properties as advanced anode material for lithium-ion batteries. Electrochim. Acta 2018, 264, 292–300. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, X.; Lu, G.; Liu, J.; He, C. Facile synthesis of MnO and nitrogen-doped carbon nanocomposites as anode material for lithium ion battery. Mater. Lett. 2014, 136, 289–291. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Li, J.; Liang, X.; Wang, J.; Bai, J.; Qian, Y. MnO@carbon core–shell nanowires as stable high-performance anodes for lithium-ion batteries. Chem.-Eur. J. 2013, 19, 11310–11319. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Wei, H.; Yan, X.; Guo, Z. Carbon-coated MnO microparticulate porous nanocomposites serving as anode materials with enhanced electrochemical performances. Nano Energy 2014, 9, 41–49. [Google Scholar] [CrossRef]
- Su, J.; Liang, H.; Gong, X.-N.; Lv, X.-Y.; Long, Y.-F.; Wen, Y.-X. Fast preparation of porous MnO/C microspheres as anode materials for lithium-ion batteries. Nanomaterials 2017, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-M.; Qie, L.; Shen, Y.; Sun, Y.-M.; Yuan, L.-X.; Hu, X.-L.; Zhang, W.-X.; Huang, Y.-H. Superior lithium storage performance in nanoscaled MnO promoted by N-doped carbon webs. Nano Energy 2013, 2, 412–418. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Guo, S.; Yan, C.; Lee, P.S.; Li, C. Rational design of MnO/carbon nanopeapods with internal void space for high-rate and long-life Li-ion batteries. ACS Nano 2014, 8, 6038–6046. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Tang, Y.; Ye, D.; Yan, J.; Zhou, H.; Wang, H. Tuning the morphologies of MnO/C hybrids by space constraint assembly of Mn-MOFs for high performance Li ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 5254–5262. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, Q.; Xu, C.; Zou, R.; Zhang, J.; Zhang, W.; Guan, G.; Hu, J.; Sun, Y. A new strategy to effectively alleviate volume expansion and enhance the conductivity of hierarchical MnO@C nanocomposites for lithium ion batteries. J. Mater. Chem. A 2017, 5, 21699–21708. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhong, J.; Yang, Z. A flexible core-shell carbon layer MnO nanofiber thin film via host-guest interaction: construction, characterization, and electrochemical performances. Carbon 2018, 128, 277–286. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and synthesis of bubble-nanorod-structured Fe2O3–carbon nanofibers as advanced anode material for Li-ion batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zheng, L.; Lan, J.-L.; Yu, Y.; Yang, X. MnO nanoparticles encapsulated in carbon nanofibers with sufficient buffer space for high-performance lithium-ion batteries. Electrochim. Acta 2018, 269, 624–631. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Ju, H.S.; Kang, Y.C. Synthesis of NiO nanofibers composed of hollow nanospheres with controlled sizes by the nanoscale Kirkendall diffusion process and their electrochemical properties. ACS Appl. Mater. Interfaces 2015, 7, 25641–25647. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, X.; Li, L.; Li, B.; Cao, D.; Wu, Q.; Li, Z.; Yang, G.; Guo, B.; Niu, C. Synthesis of SnO2 versus Sn crystals within N-doped porous carbon nanofibers via electrospinning towards high-performance lithium ion batteries. Nanoscale 2016, 8, 7595–7603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, G.; Huang, Y.; Wei, C.; Yu, Y.; Sun, D.; Yu, X. MnO quantum dots embedded in carbon nanotubes as excellent anode for lithium-ion batteries. Energy Storage Mater. 2018, 10, 160–167. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite nanofibers as advanced materials for Li-ion, Li-O2 and Li-S batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef]
- Pappa, A.M.; Karagkiozaki, V.; Krol, S.; Kassavetis, S.; Konstantinou, D.; Pitsalidis, C.; Tzounis, L.; Pliatsikas, N.; Logothetidis, S. Oxygen-plasma-modified biomimetic nanofibrous scaffolds for enhanced compatibility of cardiovascular implants. Beilstein J. Nanotechnol. 2015, 6, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Niu, Q.J.; Chen, G.K.; Nie, J.; Ma, G.P. Electrooxidation of methanol on Pt Ni bimetallic catalyst supported on porous carbon nanofibers. J. Phys. Chem. C 2017, 121, 1463–1471. [Google Scholar] [CrossRef]
- Li, W.; Zeng, L.; Wu, Y.; Yu, Y. Nanostructured electrode materials for lithium-ion and sodium-ion batteries via electrospinning. Sci. China Mater. 2016, 59, 287–321. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Xu, H.; Luo, W.; Sun, Y.; Huang, Y. Encapsulation of MnO nanocrystals in electrospun carbon nanofibers as high-performance anode materials for lithium-ion batteries. Sci. Rep. 2014, 4, 4229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-G.; Zhou, Y.; Shen, Y.; Li, Y.; Zuo, Q.; Duan, Q. Fabrication, formation mechanism and the application in lithium-ion battery of porous Fe2O3 nanotubes via single-spinneret electrospinning. Electrochim. Acta 2015, 158, 105–112. [Google Scholar] [CrossRef]
- Gu, S.; Liu, Y.; Zhang, G.; Shi, W.; Liu, Y.; Zhu, J. Fe3O4/carbon composites obtained by electrospinning as an anode material with high rate capability for lithium ion batteries. RSC Adv. 2014, 4, 41179–41184. [Google Scholar] [CrossRef]
- Zhi, C.; Ting, Y.; Huimin, S.; Taihong, W.; Ming, Z.; Guozhong, C. Single nozzle electrospinning synthesized MoO2@C core shell nanofibers with high capacity and long-term stability for lithium-ion storage. Adv. Mater. Interfaces 2017, 4, 1600816. [Google Scholar]
- Li, H.; Kang, W.; Yu, Y.; Liu, J.; Qian, Y. Synthesis of bamboo-structured carbon nanotubes and honeycomb carbons with long-cycle Li-storage performance by in situ generated zinc oxide. Carbon 2012, 50, 4787–4793. [Google Scholar] [CrossRef]
- Chu, Y.; Guo, L.; Xi, B.; Feng, Z.; Wu, F.; Lin, Y.; Liu, J.; Sun, D.; Feng, J.; Qian, Y.; et al. Embedding MnO@Mn3O4 nanoparticles in an N-doped-carbon framework derived from Mn-organic clusters for efficient lithium storage. Adv. Mater. 2018, 30, 1704244. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Yang, L.Y.; Liu, W.; Zhang, Y.; Wang, H.L.; Liu, S.; Yang, R.R.; Guo, Y.Q.; Cui, Y.P. Novel hybrid anode of MnO nanoparticles and ultrathin carbon sheets for high lithium storage performance. J. Alloys Compd. 2018, 740, 375–381. [Google Scholar] [CrossRef]
- Wang, S.; Xing, Y.; Xu, H.; Zhang, S. MnO nanoparticles interdispersed in 3D porous carbon framework for high performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 12713–12718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Mu, X.; Du, J.; Wang, H.; Huang, B.; Zhou, J.; Pan, X.; Xie, E. The carbonization temperature effect on the electrochemical performance of nitrogen-doped carbon monoliths. Electrochim. Acta 2017, 242, 100–106. [Google Scholar] [CrossRef]
- Li, H.; Kang, W.; Wang, L.; Yue, Q.; Xu, S.; Wang, H.; Liu, J. Synthesis of three-dimensional flowerlike nitrogen-doped carbons by a copyrolysis route and the effect of nitrogen species on the electrocatalytic activity in oxygen reduction reaction. Carbon 2013, 54, 249–257. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Shi, J.; Liu, W.; Shi, Z.; Chen, S.; Wang, X.; Wang, H. Two-dimensional biomass-derived carbon nanosheets and MnO/carbon electrodes for high-performance Li-ion capacitors. J. Mater. Chem. A 2017, 5, 15243–15252. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, H.; Shi, S.; Liu, T.; Yang, G.; Chao, Y.; Yin, F. 2D film of carbon nanofibers elastically astricted MnO microparticles: a fexible binder-free anode for highly reversible lithium ion storage. Small 2017, 13, 1604182. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Dong, X.; Peng, L.; Kang, W.; Li, H. The Enhanced Lithium-Storage Performance for MnO Nanoparticles Anchored on Electrospun Nitrogen-Doped Carbon Fibers. Nanomaterials 2018, 8, 733. https://doi.org/10.3390/nano8090733
Zhang R, Dong X, Peng L, Kang W, Li H. The Enhanced Lithium-Storage Performance for MnO Nanoparticles Anchored on Electrospun Nitrogen-Doped Carbon Fibers. Nanomaterials. 2018; 8(9):733. https://doi.org/10.3390/nano8090733
Chicago/Turabian StyleZhang, Rui, Xue Dong, Lechao Peng, Wenjun Kang, and Haibo Li. 2018. "The Enhanced Lithium-Storage Performance for MnO Nanoparticles Anchored on Electrospun Nitrogen-Doped Carbon Fibers" Nanomaterials 8, no. 9: 733. https://doi.org/10.3390/nano8090733