Piezoelectric Effect and Electroactive Phase Nucleation in Self-Standing Films of Unpoled PVDF Nanocomposite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Process of PVDF-Based Polymers
2.2. Characterization
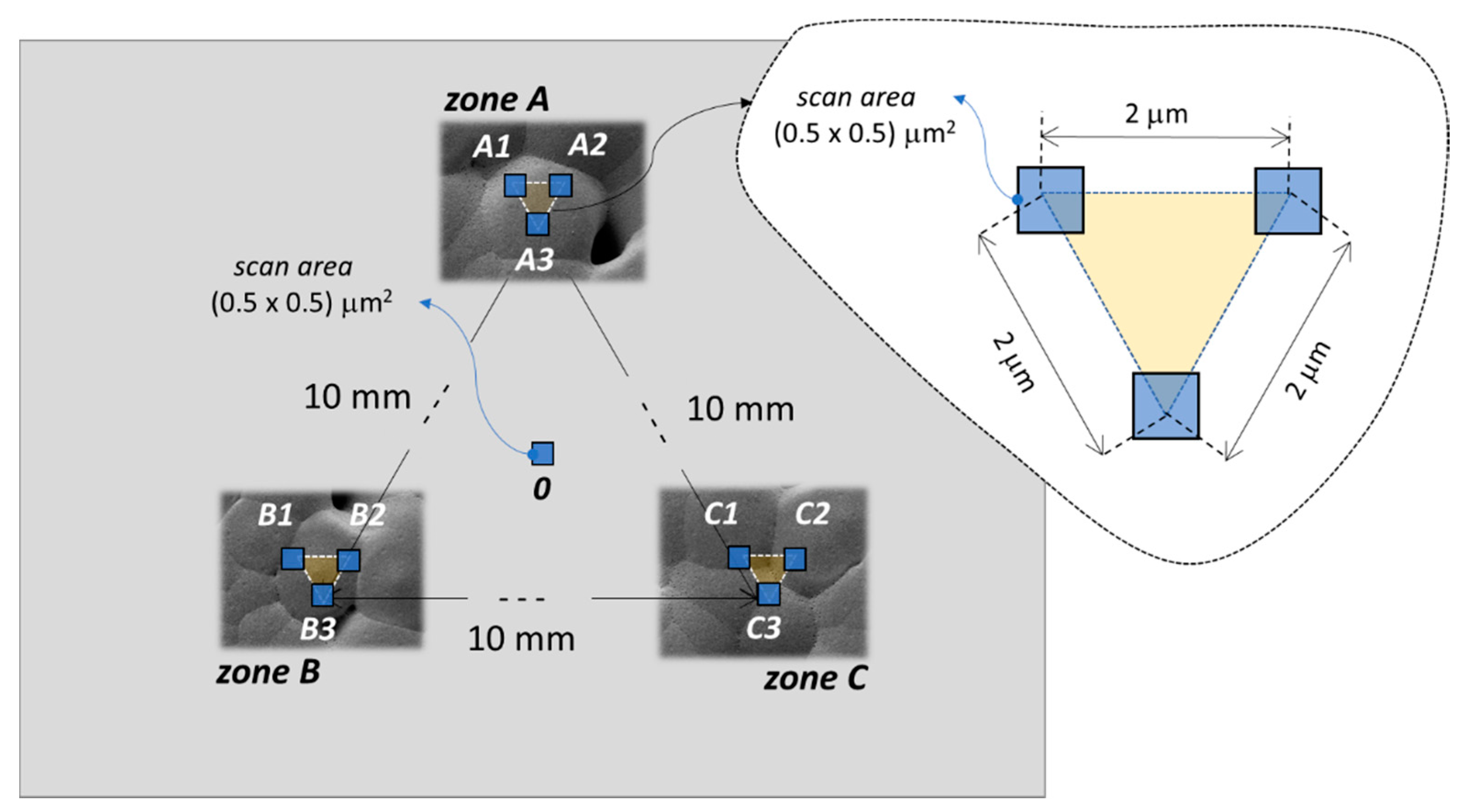
2.3. Piezoelectric Response Measurement
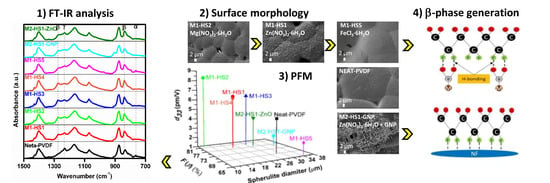
3. Results and Discussion
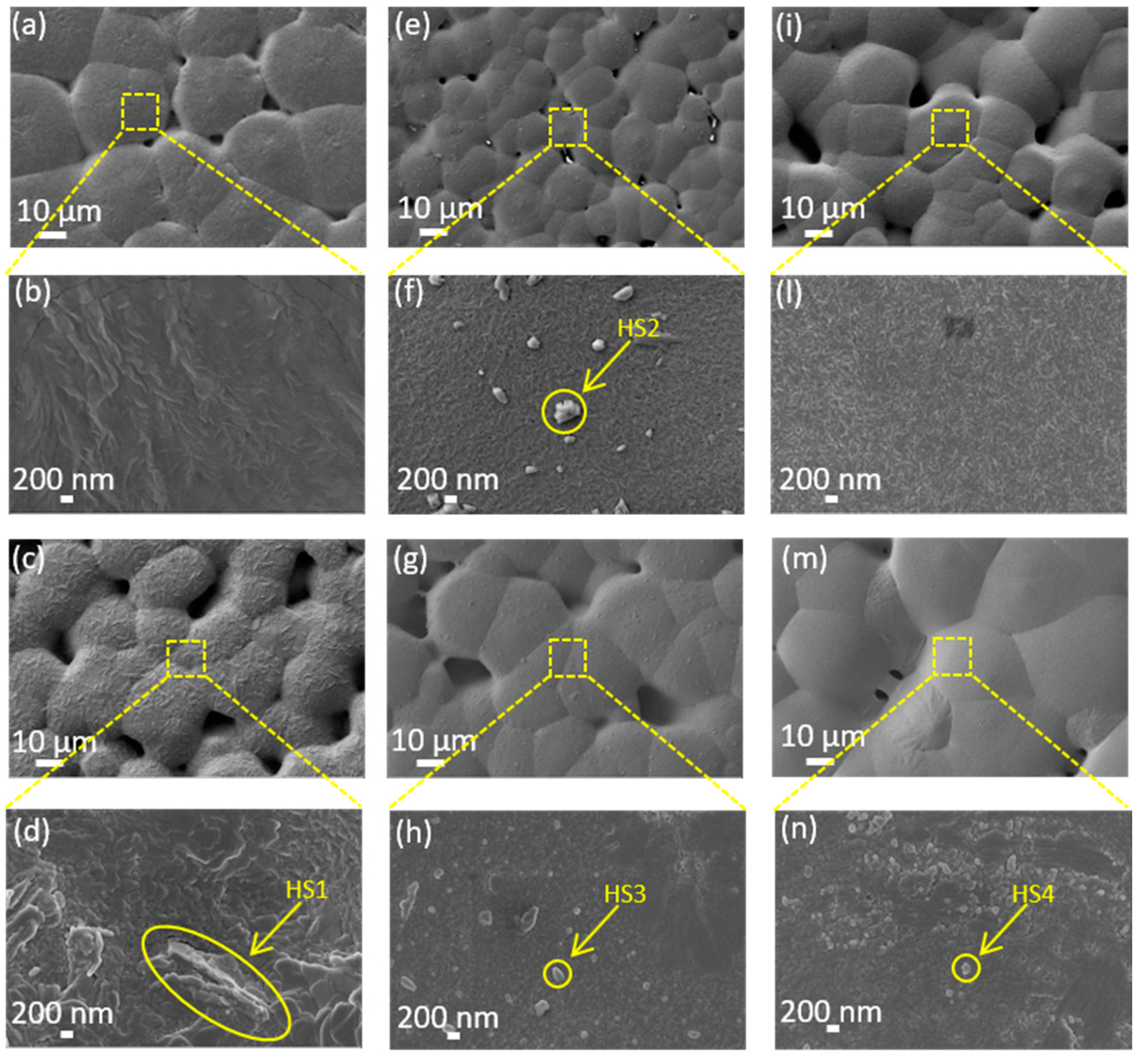
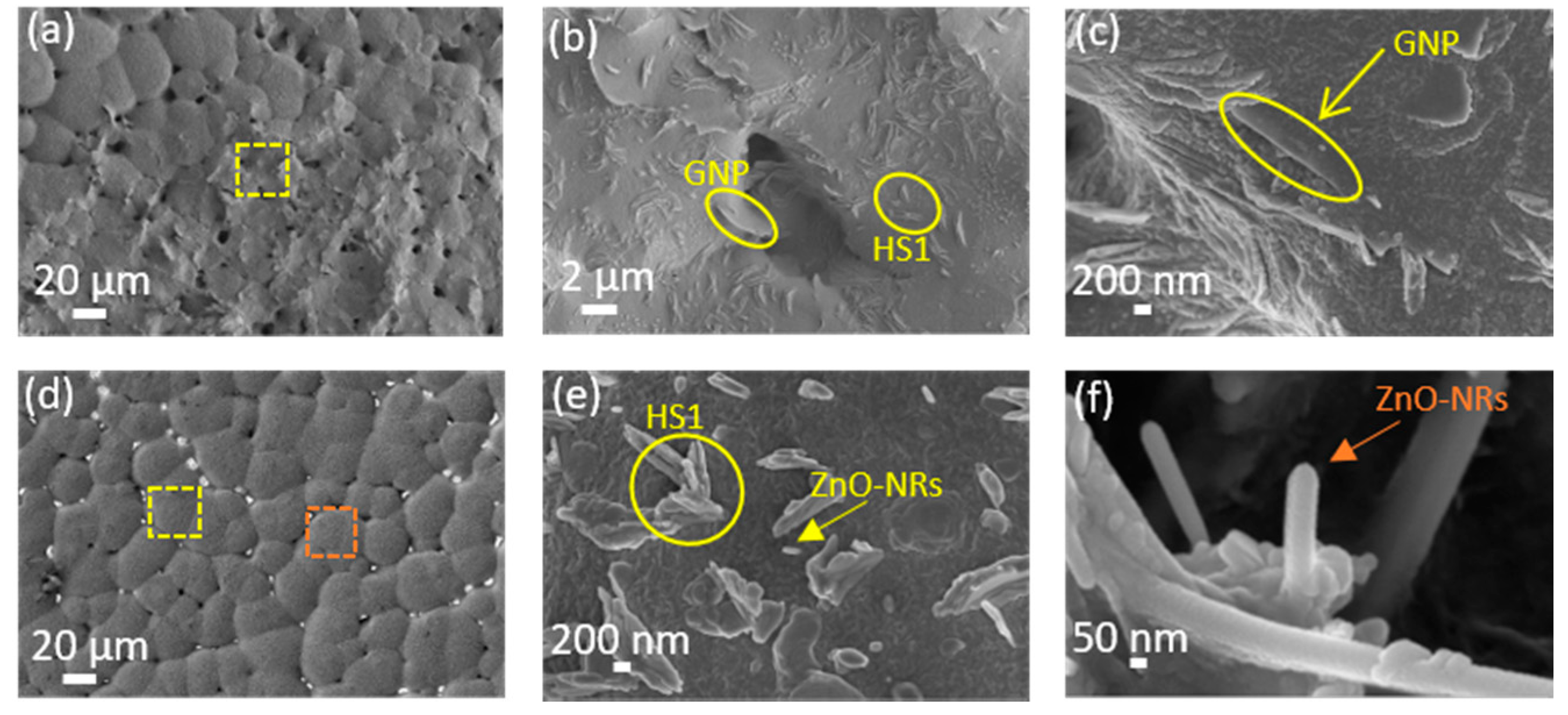
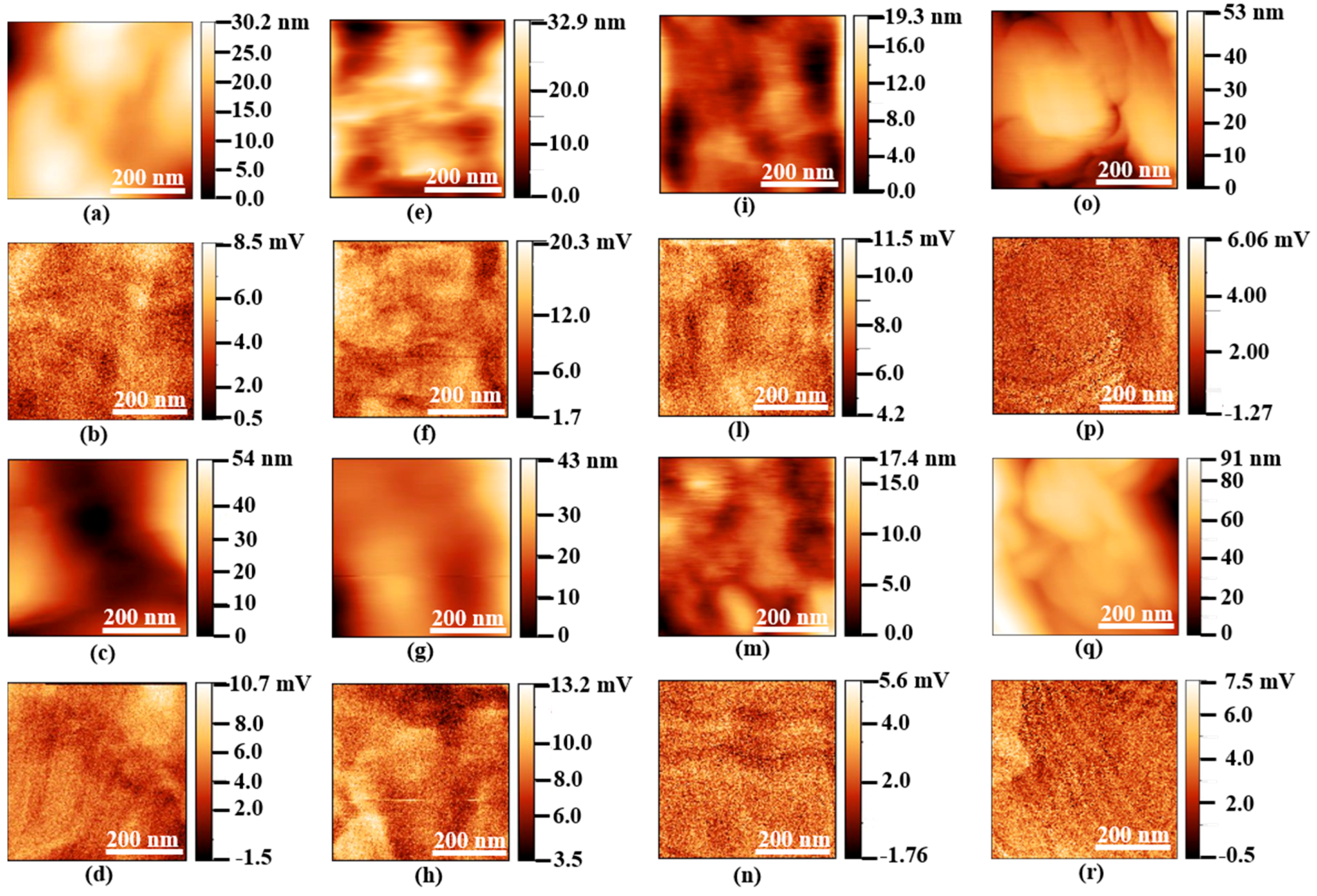
3.1. Surface Morphology
3.2. Electroactive β-Phase Enhancement
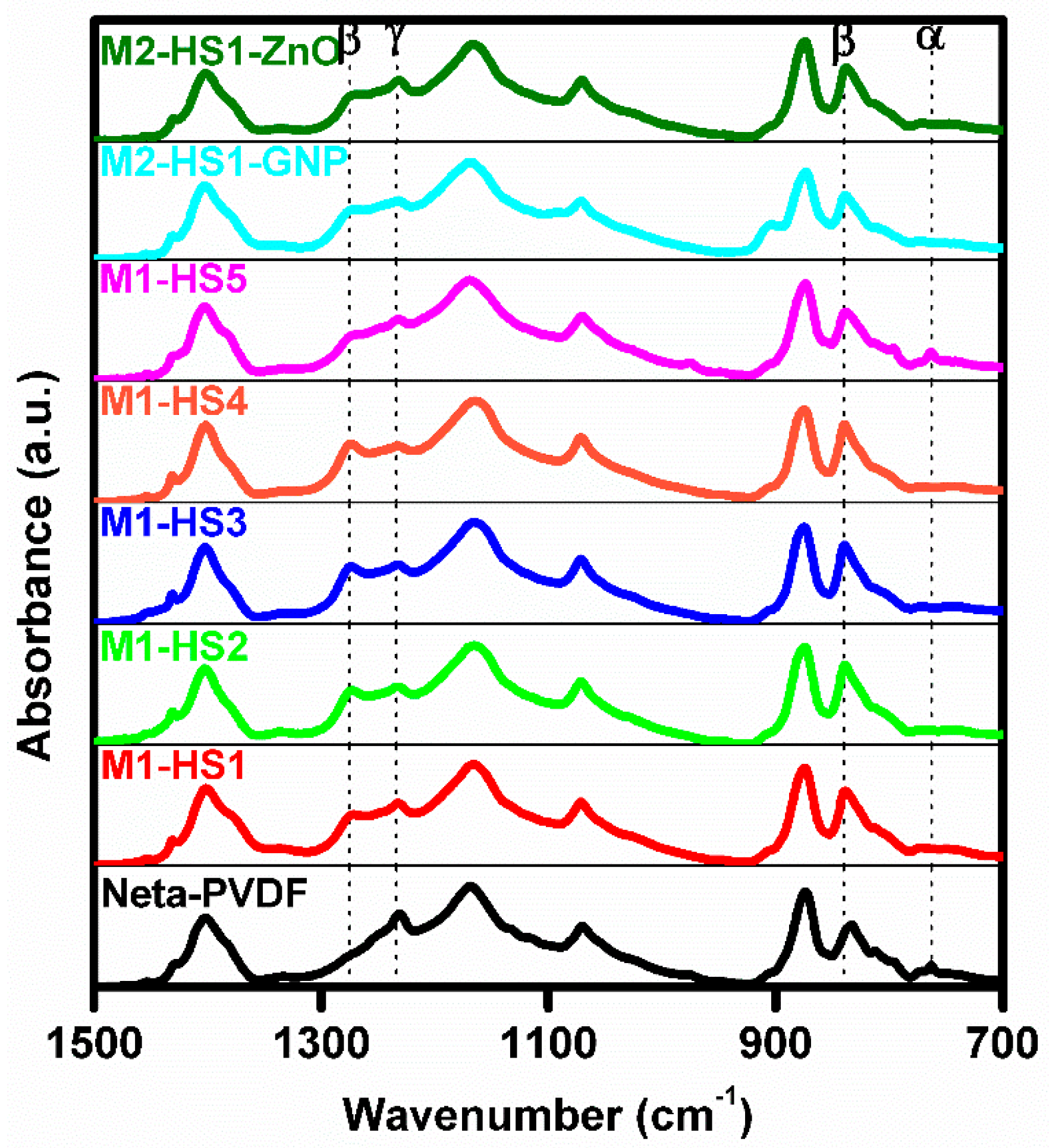
3.2.1. FT-IR Analysis
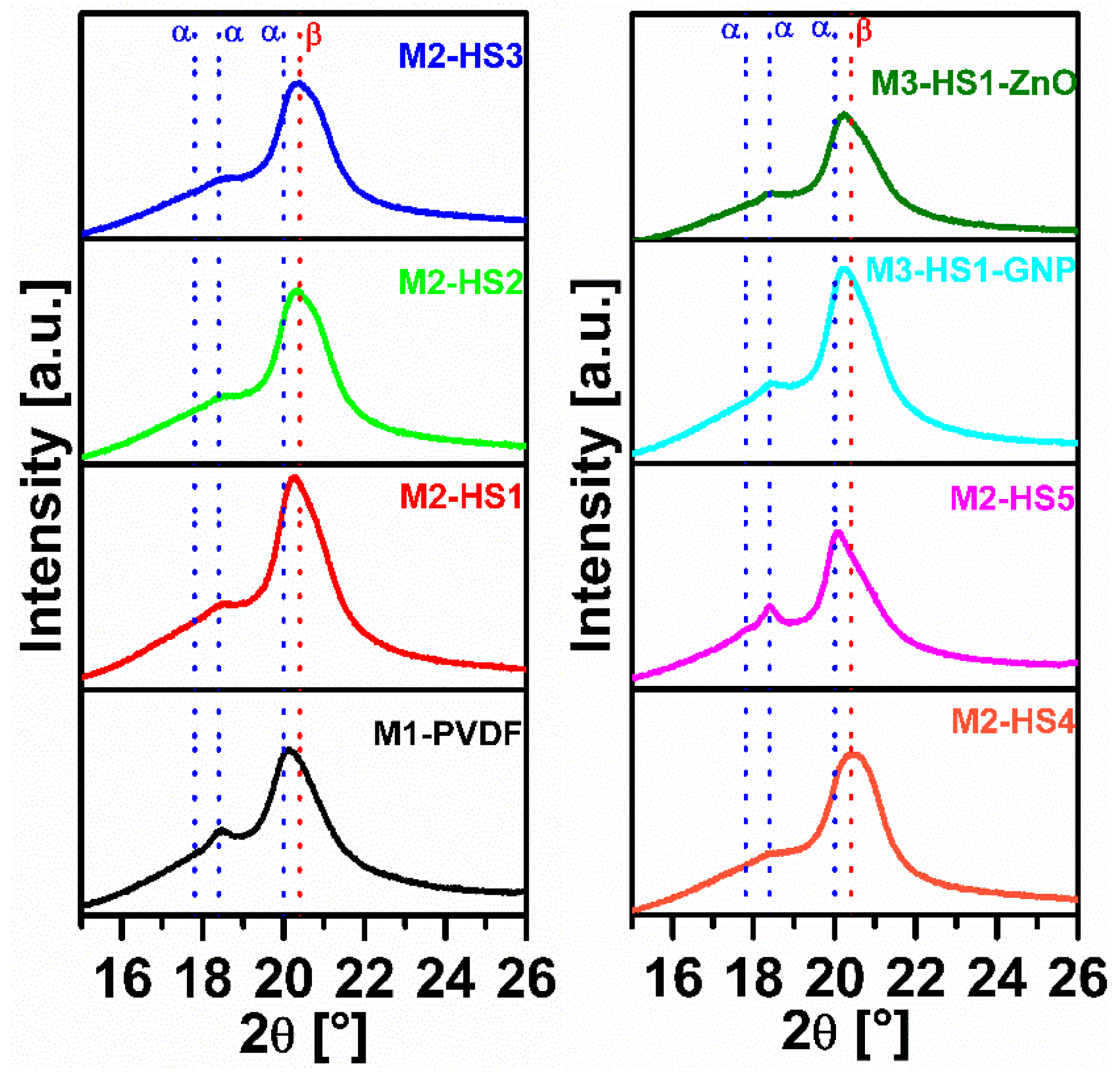
3.2.2. XRPD Analysis
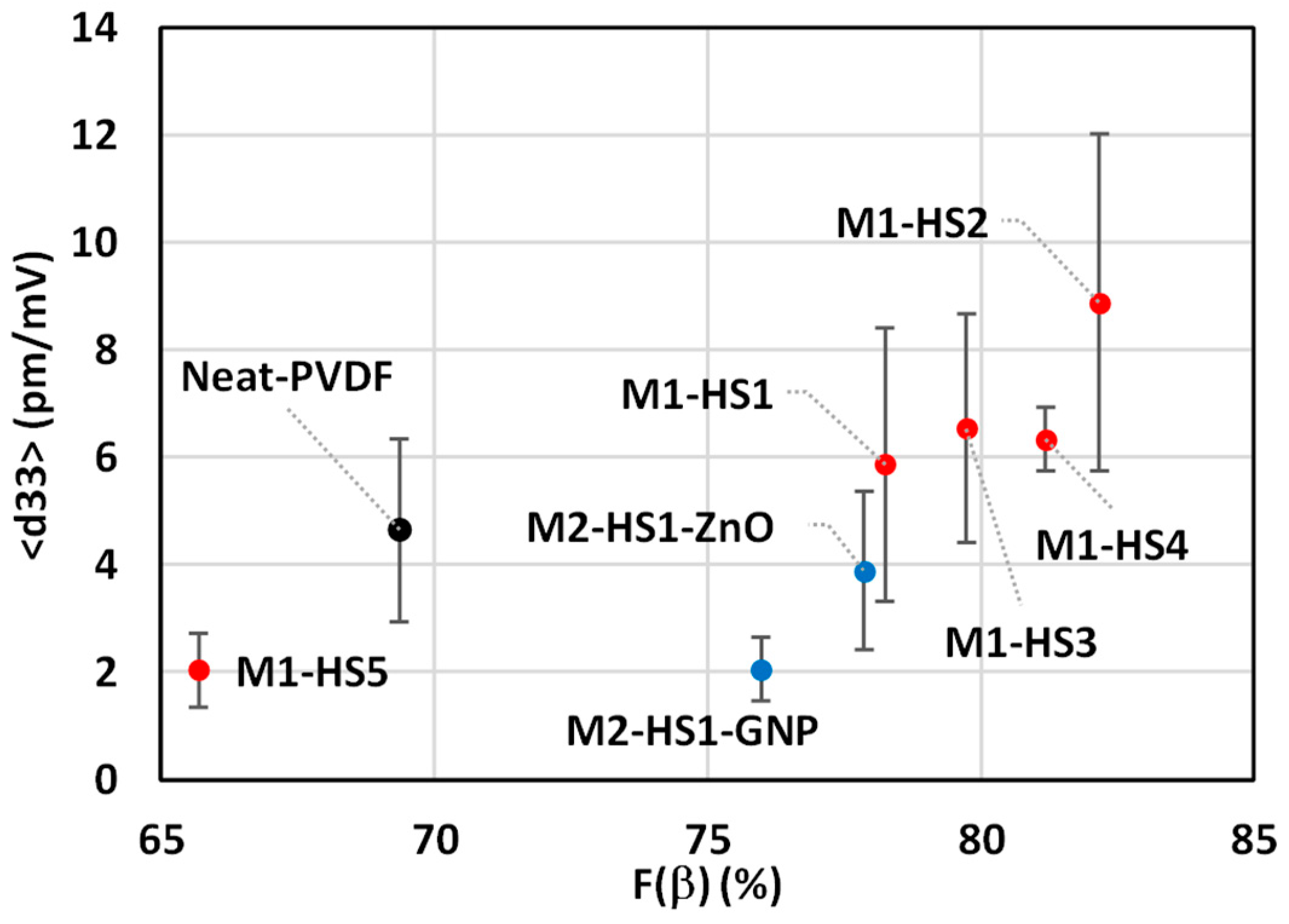
3.3. Piezoelectric Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coster, H.G.L.L.; Farahani, T.D.; Chilcott, T.C. Production and characterization of piezo-electric membranes. Desalination 2011, 283, 52–57. [Google Scholar] [CrossRef]
- Guo, D.; Zeng, F.; Dkhil, B. Ferroelectric Polymer Nanostructures: Fabrication, Structural Characteristics and Performance Under Confinement. J. Nanosci. Nanotech. 2014, 14, 2086–2100. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Garain, S.; Ghosh, S.K.; Sen, S.; Mandal, D. The preparation of γ-crystalline non-electrically poled photoluminescant ZnO-PVDF nanocomposite film for wearable nanogenerators. Nanotechnology 2016, 27, 445403. [Google Scholar] [CrossRef] [PubMed]
- Bohlén, M.; Bolton, K. Inducing the β-phase of poly(vinylidene fluoride )—A review. Ann. Rev. Nanosci. Nanotech. 2015, 1, 1–14. [Google Scholar]
- Gomes, J.; Nunes, J.S.; Sencadas, V.; Lanceros-Mendez, S.; Serrado Nunes, J.; Sencadas, V.; Lanceros-Mendez, S.; Nunes, J.S.; Sencadas, V.; Lanceros-Mendez, S. Influence of the β-phase content and degree of crystallinity on the piezo- and ferroelectric properties of poly(vinylidenefluoride). Smart Mat. Struct. 2010, 19, 065010. [Google Scholar] [CrossRef]
- Kang, S.B.; Won, S.H.; Im, M.J.; Kim, C.U.; Park, W.I.; Baik, J.M.; Choi, K.J. Enhanced piezoresponse of highly aligned electrospun poly(vinylidene fluoride) nanofibers. Nanotechnology 2017, 28, 395402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhang, M.; Rong, M.; Ruan, W. 2014 Studies on the transformation process of PVDF from α to β phase by stretching. RSC Adv. 2014, 4, 3938–3943. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Yoon, S.; Kim, K.J.; Lee, J.S. Direct Preparation of Nanoscale Thin Films of Poly(vinylidene fluoride) Containing β-Crystalline Phase by Heat-Controlled Spin Coating. Macromol. Chem. Phys. 2008, 209, 2516–2526. [Google Scholar] [CrossRef]
- Lopes, A.C.; Costa, C.M.; Tavares, C.J.; Neves, I.C.; Lanceros-Mendez, S. Nucleation of the Electroactive γ Phase and Enhancement of the Optical Transparency in Low Filler Content Poly(vinylidene)/Clay Nanocomposites. J. Phys. Chem. C 2011, 115, 18076–18082. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Deng, Y.; Xiang, Y.; Guo, L. Fabrication of radial ZnO nanowire clusters and radial ZnO/PVDF composites with enhanced dielectric properties. Adv. Funct. Mater. 2008, 18, 2584–2592. [Google Scholar] [CrossRef]
- Alam, M.M.; Ghosh, S.K.; Sarkar, D.; Sen, S.; Mandal, D. Improved dielectric constant and breakdown strength of γ-phase dominant super toughened polyvinylidene fluoride/TiO2 nanocomposite film: An excellent material for energy storage applications and piezoelectric throughput. Nanotechnology 2017, 28, 015503. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Henkel, K.; Schmeißer, D.; Dipankar, M.; Karsten, H.; Dieter, S. The electroactive β-phase formation in Poly(vinylidene fluoride) by gold nanoparticles doping. Mat. Lett. 2012, 73, 123–125. [Google Scholar] [CrossRef]
- Gan, W.C.; Abd Majid, W.H.A. Effect of TiO2 on enhanced pyroelectric activity of PVDF composite. Smart Mat. Struct. 2014, 23, 4. [Google Scholar] [CrossRef]
- Patil, R.; Ashwin, A.; Radhakrishnan, S. Novel polyaniline/{PVDF}/{BaTiO}3 hybrid composites with high piezo-sensitivity. Sensor. Actuat. A Phys. 2007, 138, 361–365. [Google Scholar] [CrossRef]
- He, X.; Yao, K. Crystallization mechanism and piezoelectric properties of solution-derived ferroelectric poly(vinylidene fluoride) thin films. Appl. Phys. Lett. 2006, 89, 112909. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, W.; Yu, W.; Zhang, Y.; Jiang, Q.; Zhao, Z. Formation mechanism of β-phase in PVDF/CNT composite prepared by the sonication method. Macromolecules 2009, 42, 8870–8874. [Google Scholar] [CrossRef]
- Kim, G.H.; Hong, S.M.; Seo, Y. Piezoelctric properties of poly(vnylidene fluoride) and carbon nanotube blends: β-phase development. Phys. Chem. Chem. Phys. 2009, 11, 10506–10512. [Google Scholar] [CrossRef] [PubMed]
- Bidsorkhi, H.C.H.C.; D’Aloia, A.G.; De Bellis, G.; Proietti, A.; Rinaldi, A.; Fortunato, M.; Ballirano, P.; Bracciale, M.P.; Santarelli, M.L.; Sarto, M.S.; et al. Nucleation effect of unmodified graphene nanoplatelets on PVDF/GNP film composites. Mat. Today Commun. 2017, 11, 163–173. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, K.-Y.; Kim, C.; Jang, J. Enhanced frequency response of a highly transparent PVDF–graphene based thin film acoustic actuator. Chem. Commun. 2013, 49, 11047. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, G.; Tamburrano, A.; Dinescu, A.; Santarelli, M.L.L.; Sarto, M.S.S. Electromagnetic properties of composites containing graphite nanoplatelets at radio frequency. Carbon 2011, 49, 4291–4300. [Google Scholar] [CrossRef]
- Tamburrano, A.; Sarasini, F.; De Bellis, G.; D’Aloia, A.G.; Sarto, M.S.S. Piezoresistive effect in graphene-based polymeric composites. Nanotechnology 2013, 24, 465702. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, M.; Bidsorkhi, H.C.C.; De Bellis, G.; Sarto, F.; Sarto, M.S.S. Piezoelectric Response of Graphene-Filled PVDF Nanocomposites Through Piezoresponse Force Microscopy (PFM). In Proceedings of the 17th IEEE International Conference on Nanotechnology, Pittsburgh, PA, USA, 25–28 July 2017. [Google Scholar]
- Fortunato, M.; Bidsorkhi, H.C.; Chandraiahgari, C.R.; De Bellis, G.; Sarto, F.; Sarto, M.S. PFM Characterization of PVDF Nanocomposite Films with Enhanced Piezoelectric Response. IEEE Trans. Nanotech. 2018, 17, 955–961. [Google Scholar] [CrossRef]
- Chandraiahgari, C.R.; De Bellis, A.; Martinelli, G.; Bakry, A.; Tamburrano, A.; Sarto, M.S. Nanofiller Induced Electroactive Phase formation in Solution Derived Poly(Vinylidene Fluoride) Polymer Composites. In Proceedings of the IEEE International Conference on Nanotechnology, Rome, Italy, 27–30 July 2015. [Google Scholar]
- Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Balijepalli, S.K.; Kaciulis, S.; Caneve, L.; Sarto, F.; Sarto, M.S. Synthesis and characterization of ZnO nanorods with a narrow size distribution. RSC Adv. 2015, 5, 49861–49870. [Google Scholar] [CrossRef]
- Fortunato, M.; Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Soltani, P.; Kaciulis, S.; Caneve, L.; Sarto, F.; Sarto, M.S. Piezoelectric Thin Films of ZnO-Nanorods/ Nanowalls Grown by Chemical Bath Deposition. IEEE Trans. Nanotech. 2018, 17, 311–319. [Google Scholar] [CrossRef]
- Thakur, P.; Kool, A.; Bagchi, B.; Hoque, N.A.; Das, S.; Nandy, P. The role of cerium(iii)/yttrium(iii) nitrate hexahydrate salts on electroactive β phase nucleation and dielectric properties of poly(vinylidene fluoride) thin films. RSC Adv. 2015, 5, 28487–28496. [Google Scholar] [CrossRef]
- Jana, S.; Garain, S.; Sen, S.; Mandal, D. The influence of hydrogen bonding on the dielectric constant and the piezoelectric energy harvesting performance of hydrated metal salt mediated {PVDF} films. Phys. Chem. Chem. Phys. 2015, 17, 17429–17436. [Google Scholar] [CrossRef] [PubMed]
- Soergel, E. Piezoresponse Force Microscopy (PFM). J. Phys. D 2011, 44, 464003. [Google Scholar] [CrossRef]
- Zheng, T.; Yue, Z.; Wallace, G.G.; Du, Y.; Martins, P.; Lanceros-Mendez, S.; Higgins, M.J. Local probing of magnetoelectric properties of PVDF/Fe3O4 electrospun nanofibers by piezoresponse force microscopy. Nanotechnology 2017, 28, 065707. [Google Scholar] [CrossRef] [PubMed]
- Paliotta, L.; De Bellis, G.; Tamburrano, A.; Marra, F.; Rinaldi, A.; Balijepalli, S.K.; Kaciulis, S.; Sarto, M.S. Highly conductive multilayer-graphene paper as a flexible lightweight electromagnetic shield. Carbon 2015, 89, 260–271. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. Poly. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Denning, D.; Guyonnet, J.; Rodriguez, B.J. Applications of piezoresponse force microscopy in materials research: from inorganic ferroelectrics to biopiezoelectrics and beyond. Int. Mat. Rev. 2016, 61, 46–70. [Google Scholar] [CrossRef]
- Garain, S.; Kumar Sinha, T.; Adhikary, P.; Henkel, K.; Sen, S.; Ram, S.; Sinha, C.; Schmeißer, D.; Mandal, D. Self-poled transparent and flexible UV light-emitting cerium complex-PVDF composite: A high-performance nanogenerator. ACS Appl. Mater. Inter. 2015, 7, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, R.; Cestari, M.; Gregorio, R.; Cestari, M.; Gregorio, R.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. Poly. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Vanysek, P. Electrochemical series. In Handbook of Chemistry and Physics; CRC Press: Cleveland, OH, USA, 2000; pp. 1–13. [Google Scholar]
- Nunes, J.S.; Wu, A.; Gomes, J.; Sencadas, V.; Vilarinho, P.M.; Lanceros-Mendez, S.; Serrado Nunes, J.; Wu, A.; Gomes, J.; Sencadas, V.; et al. Relationship between the microstructure and the microscopic piezoelectric response of the α- and β-phases of poly(vinylidene fluoride). Appl. Phys. A 2009, 95, 875–880. [Google Scholar]
- Gregorio, R.J.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Bdikin, I.K.; Silibin, M.V.; Karpinsky, D.V.; Kopyl, S.A.; Goncalves, G.; Sapronova, A.V.; Kuznetsova, T.; Bystrova, V.V. Graphene/graphene oxide and polyvinylidene fluoride polymer ferroelectric composites for multifunctional applications. Ferroelectrics 2017, 509, 124–142. [Google Scholar] [CrossRef]



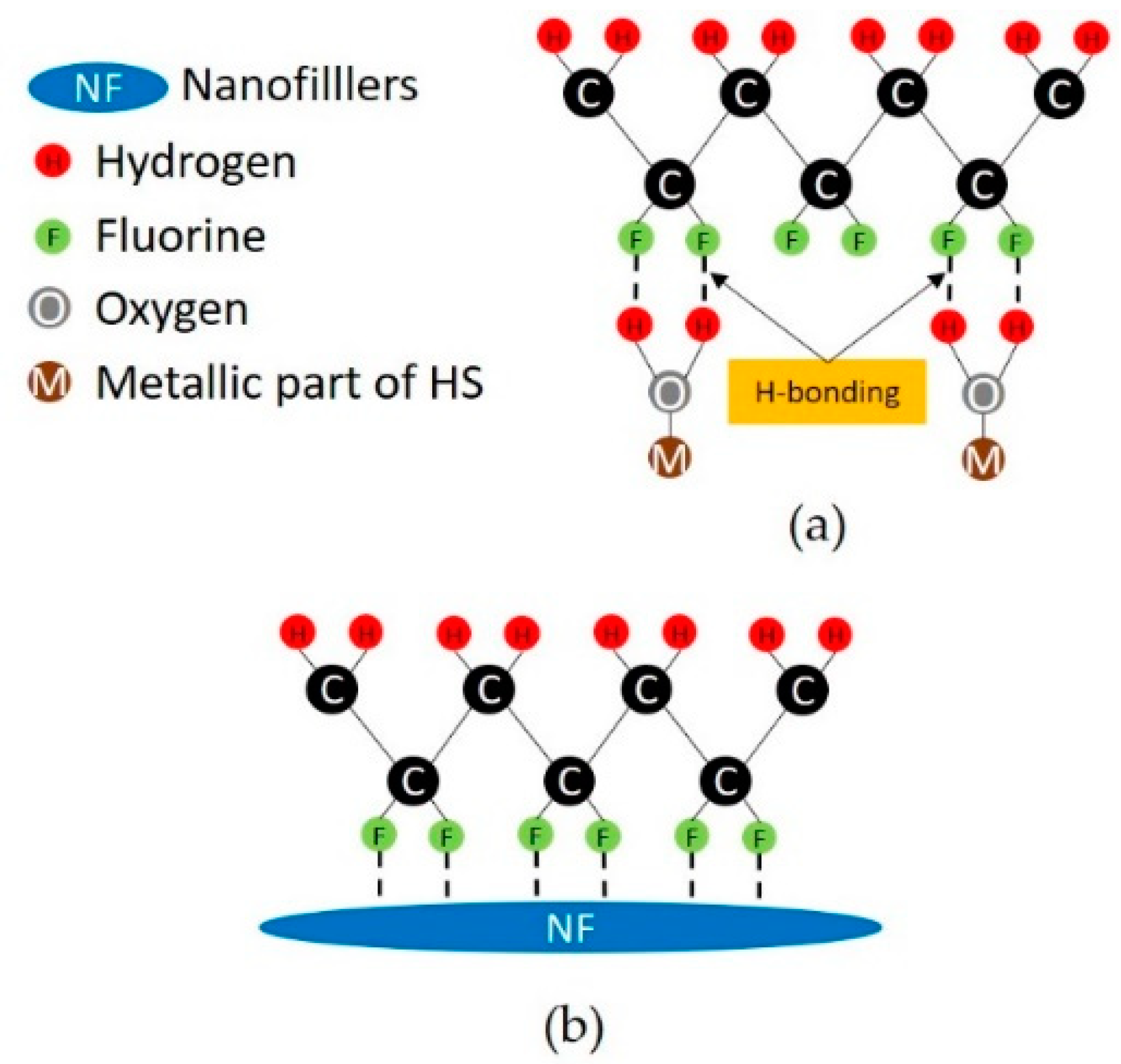
| Sample | Production Method | GNPs (wt %) | ZnO NRs (wt %) | HMS (0.2 wt %) |
|---|---|---|---|---|
| Neat-PVDF | - | - | - | - |
| M1-HS1 | M1 | - | - | Zn(NO3)2·6H2O |
| M1-HS2 | M1 | - | - | Mg(NO3)2·6H2O |
| M1-HS3 | M1 | - | - | MgCl2·6H2O |
| M1-HS4 | M1 | - | - | AlCl3·6H2O |
| M1-HS5 | M1 | - | - | FeCl3·6H2O |
| M2-HS1-GNP | M2 | 0.1 | - | Zn(NO3)2·6H2O |
| M2-HS1-ZnO | M2 | - | 0.1 | Zn(NO3)2·6H2O |
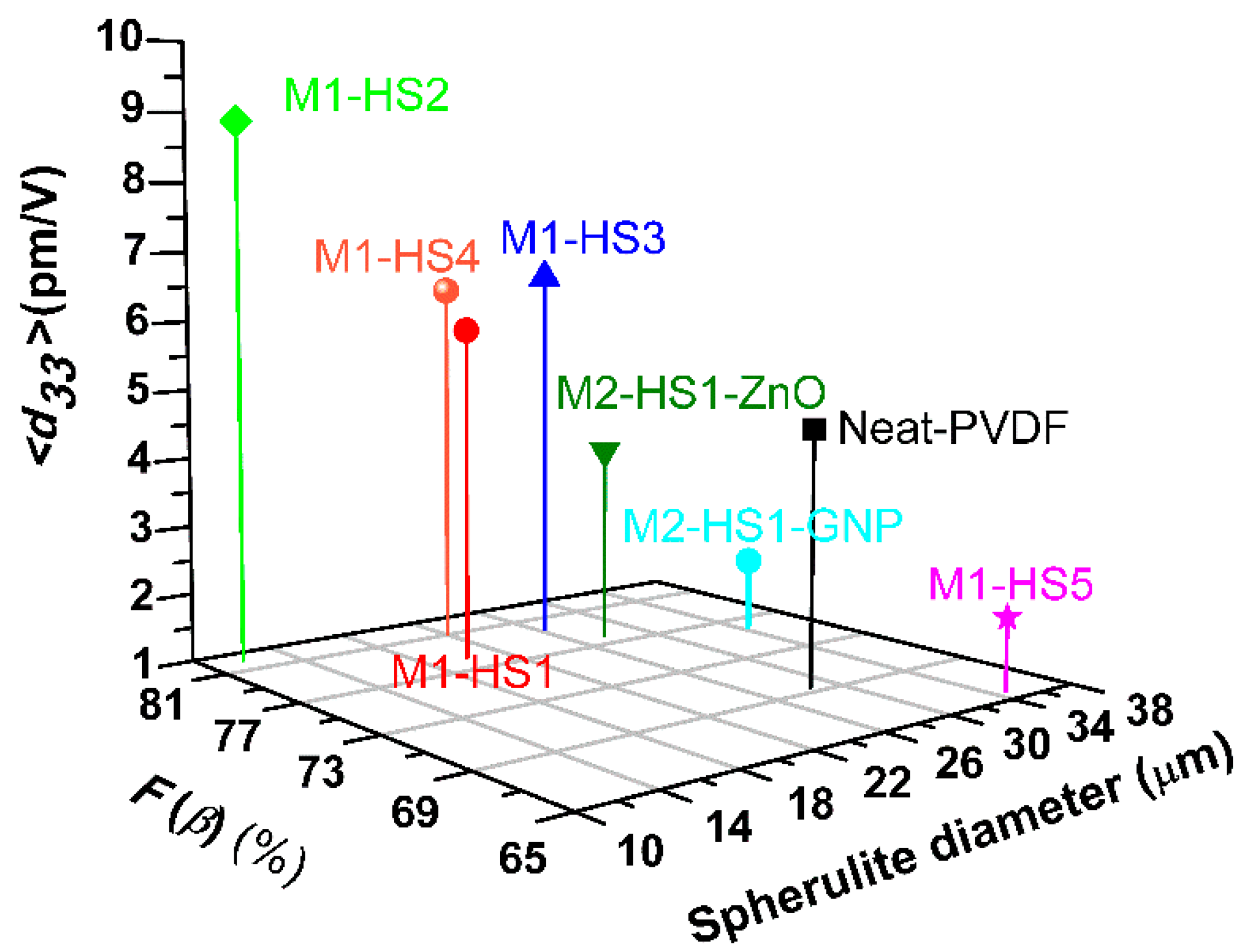
| Sample | HMS Type (0.2 wt %) | Spherulite Diameter (µm) | (%) | (pm/V) |
|---|---|---|---|---|
| Neat-PVDF | - | 28.58 ± 4.56 | 69.36 | 4.65± 1.70 |
| M1-HS1 | Zn(NO3)2·6H2O | 20.09 ± 5.33 | 78.25 | 5.87 ± 2.54 |
| M1-HS2 | Mg(NO3)2·6H2O | 11.87 ± 3.74 | 82.17 | 8.88 ± 3.14 |
| M1-HS3 | MgCl2·6H2O | 26.37 ± 5.17 | 79.73 | 6.54 ± 2.13 |
| M1-HS4 | AlCl3·6H2O | 22.19 ± 4.59 | 81.18 | 6.34 ± 0.60 |
| M1-HS5 | FeCl3·6H2O | 34.84 ± 4.36 | 65.70 | 2.04 ± 0.69 |
| M2-HS1-GNP | Zn(NO3)2·6H2O | 34.55 ± 4.67 | 75.98 | 2.05 ± 0.60 |
| M2-HS1-ZnO | Zn(NO3)2·6H2O | 27.79 ± 5.50 | 77.87 | 3.89 ± 1.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, M.; Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Sarto, F.; Tamburrano, A.; Sarto, M.S. Piezoelectric Effect and Electroactive Phase Nucleation in Self-Standing Films of Unpoled PVDF Nanocomposite Films. Nanomaterials 2018, 8, 743. https://doi.org/10.3390/nano8090743
Fortunato M, Chandraiahgari CR, De Bellis G, Ballirano P, Sarto F, Tamburrano A, Sarto MS. Piezoelectric Effect and Electroactive Phase Nucleation in Self-Standing Films of Unpoled PVDF Nanocomposite Films. Nanomaterials. 2018; 8(9):743. https://doi.org/10.3390/nano8090743
Chicago/Turabian StyleFortunato, Marco, Chandrakanth Reddy Chandraiahgari, Giovanni De Bellis, Paolo Ballirano, Francesca Sarto, Alessio Tamburrano, and Maria Sabrina Sarto. 2018. "Piezoelectric Effect and Electroactive Phase Nucleation in Self-Standing Films of Unpoled PVDF Nanocomposite Films" Nanomaterials 8, no. 9: 743. https://doi.org/10.3390/nano8090743