Inhibition of Wild Enterobacter cloacae Biofilm Formation by Nanostructured Graphene- and Hexagonal Boron Nitride-Coated Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Transfer
2.2. Characterization SLG and h-BN
2.3. Strain Isolation
2.4. Biofilm Formation Microtiter Assay—Biofilm Growth over Time
2.5. Colored Staining in Bright Light Microscopy—Effect of Media Replacement on Biofilm Growth
2.6. Inhibition of Biofilm Growth in Coated Surfaces
2.7. Scanning Electron Microscopy Images
2.8. Epifluorescence Essay
2.9. Viability of Planktonic Cells Assay—State of Non-Attached Bacteria
3. Results and Discussion
3.1. E. cloacae Biofilm Growth
3.2. Morphology of E. cloacae Biofilm at Reversible and Irreversible Stages
3.3. Characterization of Nanostructured Coated Samples
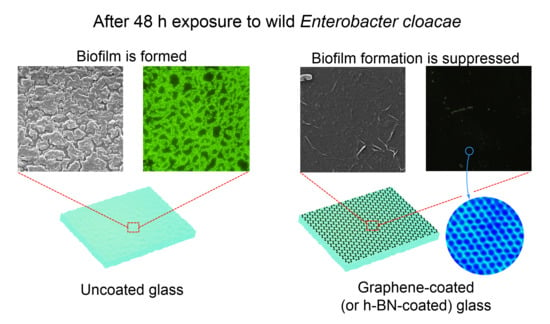
3.4. Biofilm Formation on h-BN- and Graphene-Coated Samples
3.5. Nanostructured Coating Effects on Surface Energy and Electrostatic Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Simões, M.; Simoes, L.C.; Vieira, M.J. A Review of Current and Emergent Biofilm Control Strategies. Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.J.; Shi, Q.S.; Ouyang, Y.S.; Chen, Y.B.; Hu, W.F. Efficacy of Metal Ions and Isothiazolones in Inhibiting Enterobacter cloacae BF-17 Biofilm Formation. Can. J. Microbiol. 2013, 60, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, A.K. Optimization of Cultural Conditions for Production of Extracellular Polymeric Substances (EPS) by Serpentine rhizobacterium Cupriavidus pauculus KPS 201. J. Polym. 2013, 2013, 692374. [Google Scholar] [CrossRef]
- Coenye, T.; Nelis, H.J. In Vitro and in Vivo Model Systems to Study Microbial Biofilm Formation. J. Microbiol. Methods 2010, 83, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, Y.K.; Kim, H.J.; Scheicher, R.H.; Cho, J.H. Physisorption of DNA nucleobases on h-BN and graphene: vdW-corrected DFT calculations. J. Phys. Chem. C 2013, 117, 13435–13441. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should We Stay or Should We Go: Mechanisms and Ecological Consequences for Biofilm Dispersal. Nat. Rev. Microbiol. 2011, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Vickery, K.; Walker, J.D.; deLancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached Cells, Biofilms and Biocide Susceptibility: Implications for Hospital Cleaning and Disinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial Biofilm Development as a Multicellular Adaptation: Antibiotic Resistance and New Therapeutic Strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Nyenje, M.E.; Green, E.; Ndip, R.N. Evaluation of the Effect of Different Growth Media and Temperature on the Suitability of Biofilm Formation by Enterobacter cloacae Strains Isolated from Food Samples in South Africa. Molecules 2013, 18, 9582–9593. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Beściak, G.; Surmacz-Górska, J. Biofilm as a Basic Life Form of Bacteria. In Proceedings of the Polish-Swedish-Ukrainian Seminar, Krakow, Poland, 17–19 October 2011; pp. 1–8. [Google Scholar]
- Brandes, J.; Kuhajek, J.M.; Goodwin, E.; Wood, S.A. Molecular Characterisation and Co-cultivation of Bacterial Biofilm Communities Associated with the Mat-Forming Diatom Didymosphenia geminata. Microb. Ecol. 2016, 72, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Pereira, A.M.; Pereira, M.C.; Simões, M.; Melo, L.F. Biofilm Control with New Microparticles with Immobilized Biocide. Heat Transf. Eng. 2013, 34, 174–179. [Google Scholar] [CrossRef]
- Ricart, M.; Guasch, H.; Alberch, M.; Barceló, D.; Bonnineau, C.; Geiszinger, A.; Ferrer, J.; Ricciardi, F.; Romaní, A.M.; Morin, S.; et al. Triclosan Persistence Through Wastewater Treatment Plants and Its Potential Toxic Effects on River Biofilms. Aquat. Toxicol. 2010, 100, 346–353. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary Ammonium Compounds (QACs): A Review on Occurrence, Fate and Toxicity in The Environment. Sci. Total. Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef]
- Davison, W.M.; Pitts, B.; Stewart, P.S. Spatial and Temporal Patterns of Biocide Action against Staphylococcus epidermidis Biofilms. Antimicrob. Agents Chemother. 2010, 54, 2920–2927. [Google Scholar] [CrossRef]
- Cos, P.; Tote, K.; Horemans, T.; Maes, L. Biofilms: An Extra Hurdle for Effective Antimicrobial Therapy. Curr. Pharm. Des. 2010, 16, 2279–2295. [Google Scholar] [CrossRef]
- Cappitelli, F.; Polo, A.; Villa, F. Biofilm Formation in Food Processing Environments is Still Poorly Understood and Controlled. Food Eng. Rev. 2014, 6, 29–42. [Google Scholar] [CrossRef]
- Musico, Y.L.; Santos, C.M.; Dalida, M.L.; Rodrigues, D.F. Surface modification of membrane filters using graphene and graphene oxide-based nanomaterials for bacterial inactivation and removal. ACS Sustain. Chem. Eng. 2014, 2, 1559–1565. [Google Scholar] [CrossRef]
- Rizzello, L.; Cingolani, R.; Pompa, P.P. Nanotechnology Tools for Antibacterial Materials. Nanomedicine 2013, 8, 807–821. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Elimelech, M. Toxic Effects of Single-walled Carbon Nanotubes in the Development of E. coli Biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The Controversial Antibacterial Activity of Graphene-Based Materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Chen, L.; Hu, P.; Zhang, L.; Huang, S.; Luo, L.; Huang, C. Toxicity of Graphene Oxide and Multi-walled Carbon Nanotubes Against Human Cells and Zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver Nanoparticles Impede the Biofilm Formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M. A review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antibacterial Activity of Nanomaterials. Nanomaterials 2018, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; Oukarroum, A.; Pirastru, L.; Sirois, L.; Gerson, M.W.; Popovic, R. Evaluation of Copper Oxide Nanoparticles Toxicity Using Chlorophyll Fluorescence Imaging in Lemna Gibba. J. Bot. 2010, 2010, 763142. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, F.; Jiang, P.; Tanaka, T. Is Graphene Oxide an Insulating Material? In Proceedings of the 2013 IEEE International Conference on Solid Dielectrics (ICSD), Bologna, Italy, 30 June–4 July 2013; pp. 904–907. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing Bacterial Interaction with Copper Surfaces Through Graphene and Hexagonal-boron Nitride Coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A Nanomolecular Approach to Decrease Adhesion of Biofouling-producing Bacteria to Graphene-coated Material. J. Nanobiotechnol. 2015, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, F.; Sun, M. Graphene, Hexagonal Boron Nitride, and Their Heterostructures: Properties and Applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Gentil, D.; del Campo, V.; Henrique Rodrigues da Cunha, T.; Henríquez, R.; Häberle, P.; Garín, C.; Ramírez, C.; Fuentes, R.; et al. The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions. Materials 2017, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ci, L.; Lu, H.; Sorokin, P.B.; Jin, C.; Ni, J.; Kvashnin, A.G.; Kvashnin, D.G.; Lou, J.; Yakobson, B.I.; et al. Large Scale Growth and Characterization of Atomic Hexagonal Boron Nitride Layers. Nano Lett. 2010, 10, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.L.; Zhang, K.; Li, H.; Juang, Z.Y.; et al. Synthesis of Few-layer Hexagonal Boron Nitride Thin film by Chemical Vapor Deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Mezzatesta, M.L.; Gona, F.; Stefani, S. Enterobacter cloacae Complex: Clinical Impact and Emerging Antibiotic Resistance. Future Microbiol. 2012, 7, 887–902. [Google Scholar] [CrossRef]
- Bruinsma, G.M.; Van der Mei, H.C.; Busscher, H.J. Bacterial Adhesion to Surface Hydrophilic and Hydrophobic Contact Lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Ramírez, C.; Gallegos, I.; Ihl, M.; Bifani, V. Study of Contact Angle, Wettability and Water Vapor Permeability in Carboxymethylcellulose (CMC) Based Film with Murta Leaves (Ugni molinae Turcz) Extract. J. Food Eng. 2012, 109, 424–429. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 47, 10–11. [Google Scholar] [CrossRef]
- Nishijima, K.A.; Couey, H.M.; Alvarez, A.M. Internal yellowing, a bacterial disease of papaya fruits caused by Enterobacter cloacae. Plant Dis. 1987, 71, 1029–1034. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Chelius, M.K.; Triplett, E.W. The Diversity of Archaea and Bacteria in Association with The Roots of Zea mays L. Microb. Ecol. 2001, 41, 252–263. [Google Scholar] [CrossRef]
- Rochelle, P.A.; Fry, J.C.; John Parkes, R.; Weightman, A.J. DNA Extraction for 16S rRNA Gene Analysis to Determine Genetic Diversity in Deep Sediment Communities. FEMS Microbiol. Lett. 1992, 100, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R.; Freger, V.; Lee, J.H.; Kim, Y.G.; Lee, J.; Herzberg, M. Should I Stay or Should I Go? Bacterial Attachment vs. Biofilm Formation on Surface-modified Membranes. Biofouling 2014, 30, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm Formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar]
- Zhao, Q.; Liu, Y.; Wang, C.; Wang, S.; Peng, N.; Jeynes, C. Bacterial Adhesion on Ion-implanted Stainless Steel Surfaces. Appl. Surf. Sci. 2007, 253, 8674–8681. [Google Scholar] [CrossRef]
- Gao, L.; Pan, X.; Zhang, D.; Mu, S.; Lee, D.J.; Halik, U. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Res. 2015, 1, 51–58. [Google Scholar] [CrossRef]
- Rayner, J.; Veeh, R.; Flood, J. Prevalence of microbial biofilms on selected fresh produce and household surfaces. Int. J. Food Microbiol. 2004, 95, 29–39. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2011, 22. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Tegou, E.; Magana, M.; Katsogridaki, A.E.; Ioannidis, A.; Raptis, V.; Jordan, S.; Chatzipanagiotou, S.; Chatzandroulis, S.; Ornelas, C.; Tegos, G.P. Terms of Endearment: Bacteria Meet Graphene Nanosurfaces. Biomaterials 2016, 89, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Shellenberger, K.; Logan, B.E. Effect of Molecular Scale Roughness of Glass Beads on Colloidal and Bacterial Deposition. Environ. Sci. Technol. 2002, 36, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Logan, B.E. Bacterial Adhesion to Glass and Metal-oxide Surfaces. Colloids Surf. B 2004, 36, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yankowitz, M.; Xue, J.; Cormode, D.; Sanchez-Yamagishi, J.D.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P.; Jacquod, P.; LeRoy, B.J. Emergence of Superlattice Dirac Points in Graphene on Hexagonal Boron Nitride. Nat. Phys. 2012, 8, 382. [Google Scholar] [CrossRef]
- Liu, L.; Park, J.; Siegel, D.A.; McCarty, K.F.; Clark, K.W.; Deng, W.; Basile, L.; Idrobo, J.C.; Li, A.P.; Gu, G. Heteroepitaxial Growth of Two-dimensional Hexagonal Boron Nitride Templated by Graphene Edges. Science 2014, 343, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of Multi-layer Graphene Films on Copper Tape by Atmospheric Pressure Chemical Vapor Deposition Method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035012. [Google Scholar]
- Caneva, S.; Weatherup, R.S.; Bayer, B.C.; Blume, R.; Cabrero-Vilatela, A.; Braeuninger-Weimer, P.; Martin, M.B.; Wang, R.; Baehtz, C.; Schloegl, R.; et al. Controlling Catalyst Bulk Reservoir Effects for Monolayer Hexagonal Boron Nitride CVD. Nano Lett. 2016, 16, 1250–1261. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Pan, Z.; Fu, L.; Zhang, C.; Dai, B.; Liu, Z. The Origin of Wrinkles on Transferred Graphene. Nano Res. 2011, 4, 996. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial Activity of Large-area Monolayer Graphene Film Manipulated by Charge Transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Cerca, N.; Pier, G.B.; Vilanova, M.; Oliveira, R.; Azeredo, J. Quantitative Analysis of Adhesion and Biofilm Formation on Hydrophilic and Hydrophobic Surfaces of Clinical Isolates of Staphylococcus epidermidis. Res. Microbiol. 2005, 156, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive Bacterial Cell Envelopes: The Impact on the Activity of Antimicrobial Peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Gregory, J.; Jia, X. Particle Deposition and Aggregation: Measurement, Modelling and Simulation; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Itoh, H.; Sakuma, H. Dielectric Constant of Water as a Function of Separation in a Slab Geometry: A Molecular Dynamics Study. J. Chem. Phys. 2015, 142, 184703. [Google Scholar] [CrossRef] [PubMed]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of Forces in Liquids Using a Force Microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Dupont-Gillain, C.C.; Nonckreman, C.J.; Adriaensen, Y.; Rouxhet, P.G. Fabrication of Surfaces with Bimodal Roughness through Polyelectrolyte/Colloid Assembly. In Advances in Unconventional Lithography; InTech: Vienna, Austria, 2011. [Google Scholar]
- Deshpande, A.; Bao, W.; Miao, F.; Lau, C.N.; LeRoy, B.J. Spatially Resolved Spectroscopy of Monolayer Graphene on SiO2. Phys. Rev. B 2009, 79, 205411. [Google Scholar] [CrossRef]
- Lei, W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron Nitride Colloidal Solutions, Ultralight Aerogels and Freestanding Membranes Through One-step Exfoliation and Functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef]
- Van Merode, A.E.; Pothoven, D.C.; Van Der Mei, H.C.; Busscher, H.J.; Krom, B.P. Surface Charge Influences Enterococcal Prevalence in Mixed-species Biofilms. J. Appl. Microbiol. 2007, 102, 1254–1260. [Google Scholar] [CrossRef]
- Busscher, H.J.; Weerkamp, A.H. Specific and Non-specific Interactions in Bacterial Adhesion to Solid Substrata. FEMS Microbiol. Lett. 1987, 46, 165–173. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The Wetting of Steel, DLC Coatings, Ceramics and Polymers with Oils and Water: The Importance and Correlations of Surface Energy, Surface Tension, Contact Angle and Spreading. Appl. Surf. Sci. 2014, 293, 97–108. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurob, E.; Dennett, G.; Gentil, D.; Montero-Silva, F.; Gerber, U.; Naulín, P.; Gómez, A.; Fuentes, R.; Lascano, S.; Rodrigues da Cunha, T.H.; et al. Inhibition of Wild Enterobacter cloacae Biofilm Formation by Nanostructured Graphene- and Hexagonal Boron Nitride-Coated Surfaces. Nanomaterials 2019, 9, 49. https://doi.org/10.3390/nano9010049
Zurob E, Dennett G, Gentil D, Montero-Silva F, Gerber U, Naulín P, Gómez A, Fuentes R, Lascano S, Rodrigues da Cunha TH, et al. Inhibition of Wild Enterobacter cloacae Biofilm Formation by Nanostructured Graphene- and Hexagonal Boron Nitride-Coated Surfaces. Nanomaterials. 2019; 9(1):49. https://doi.org/10.3390/nano9010049
Chicago/Turabian StyleZurob, Elsie, Geraldine Dennett, Dana Gentil, Francisco Montero-Silva, Ulrike Gerber, Pamela Naulín, Andrea Gómez, Raúl Fuentes, Sheila Lascano, Thiago Henrique Rodrigues da Cunha, and et al. 2019. "Inhibition of Wild Enterobacter cloacae Biofilm Formation by Nanostructured Graphene- and Hexagonal Boron Nitride-Coated Surfaces" Nanomaterials 9, no. 1: 49. https://doi.org/10.3390/nano9010049