Bifunctionalized Silver Nanoparticles as Hg2+ Plasmonic Sensor in Water: Synthesis, Characterizations, and Ecosafety
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. AgNPs Synthesis
2.3. AgNPs Sensing Tests
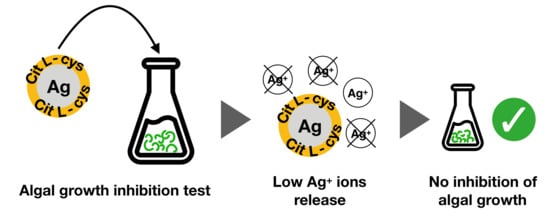
2.4. AgNPs Ecosafety Tests
3. Results and Discussion
3.1. AgNPs Synthesis and Characterizations
3.2. AgNPs Sensing Tests
3.3. SR-XPS Characterization of AgNPs and AgNPs-Hg2+
3.4. AgNPs Ecosafety
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.E.; Murphy, C.J. Applications of colloidal inorganic nanoparticles: From medicine to energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Gold nanoparticles in photonic crystals applications: A review. Materials 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical characterization of squaraine-sensitized nickel oxide cathodes deposited via screen-printing for p-type dye-sensitized solar cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Dadashi, S.; Poursalehi, R.; Delavari, H. Optical and structural properties of oxidation resistant colloidal bismuth/gold nanocomposite: An efficient nanoparticles based contrast agent for X-ray computed tomography. J. Mol. Liq. 2018, 254, 12–19. [Google Scholar] [CrossRef]
- de Angelis, R.; Venditti, I.; Fratoddi, I.; de Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Yu, C.-J.; Lin, Y.-H.; Tseng, W.-L. Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal. Chem. 2010, 82, 6830–6837. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.; Wang, T.; Ma, Z.; Su, Z. L-Cysteine functionalized gold nanoparticles for the colorimetric detection of Hg2+ induced by ultraviolet light. Nanotechnology 2010, 21, 025501. [Google Scholar] [CrossRef] [PubMed]
- Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferrato, R. Sensitivity to heavy-metal ions of unfolded fullerene quantum dots. Sensors 2017, 17, 2614. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Chen, H.-L.; Tang, C.-C.; Wu, H.-H.; Jan, J.-S. Synthesis of silica/polypeptide hybrid nanomaterials and mesoporous silica by molecular replication of sheet-like polypeptide complexes through biomimetic mineralization. J. Colloid Interface Sci. 2019, 542, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Burratti, L.; Ciotta, E.; Bolli, E.; Kaciulis, S.; Casalboni, M.; De Matteis, F.; Garzón-Manjón, A.; Scheu, C.; Pizzoferrato, R.; Prosposito, P. Fluorescence enhancement induced by the interaction of silver nanoclusters with lead ions in water. Colloid Surf. Sci. A 2019, 579, 123634. [Google Scholar] [CrossRef]
- Corsi, P.; Venditti, I.; Battocchio, C.; Meneghini, C.; Bruni, F.; Prosposito, P.; Mochi, F.; Capone, B. Designing an optimal ion adsorber on the nanoscale: A simple theoretical model for the unusual nucleation of AgNPs/Co2+–Ni2+ binary mixtures. J. Phys. Chem. C 2019, 123, 3855–3860. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Donadio, S.; Fontana, L.; Porcaro, F.; Battocchio, C.; Venditti, I.; Bracci, L.; Fratoddi, I. Negatively charged gold nanoparticles as dexamethasone carrier: Stability and citotoxic activity. RCS Adv. 2016, 6, 99016–99022. [Google Scholar]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, H.; Li, K.; Xiang, J.; Lan, T.; Zhang, Z. Fabrication of silver nanoparticle sponge leather with durable antibacterial property. J. Colloid Interface Sci. 2018, 514, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.; Nguyen, T.V.; Chu, A.D.; Tran, H.V.; Tran, L.T.; Huyn, C.D. A label-free colorimetric sensor based on silver nanoparticles directed to hydrogen peroxide and glucose. Arab. J. Chem. 2018, 11, 1134–1143. [Google Scholar] [CrossRef]
- Rinaldi, F.; del Favero, E.; Moeller, J.; Hanieh, P.N.; Passeri, D.; Rossi, M.; Angeloni, L.; Venditti, I.; Marianecci, C.; Carafa, M.; et al. Hydrophilic silver nanoparticles loaded into niosomes: Physical-chemical characterization in view of biological applications. Nanomaterials 2019, 9, 1177. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, F.; Carlini, L.; Ugolini, A.; Visaggio, D.; Luisetto, I.; Visca, P.; Fratoddi, I.; Venditti, I.; Simonelli, L.; Marini, C.; et al. Synthesis and structural characterization of silver nanoparticles stabilized with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols for antibacterial applications. Materials 2016, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Katok, K.V.; DWhitby, R.L.; Fukuda, T.; Maekawa, T.; Bezverkhyy, I.; Mikhalovsky, S.V.; Cundy, A.B. Hyperstoichiometric interaction between silver and mercury at the nanoscale. Angew. Chem. Int. Ed. 2012, 51, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Bothra, S.; Solanki, J.N.; Sahoo, S.K. Functionalized silver nanoparticles as chemosensor for pH, Hg2+ and Fe3+ in aqueous medium. Sens. Actuators B Chem. 2013, 188, 937–943. [Google Scholar] [CrossRef]
- Brandon, M.P.; Ledwith, D.M.; Kelly, J.M. Preparation of saline-stable, silica-coated triangular silver nanoplates of use for optical sensing. J. Colloid Interface Sci. 2014, 415, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; De Matteis, F.; Casciardi, S.; et al. Interaction of colloidal silver nanoparticles with Co2+ and Ni2+ in water for sensing application. Nanomaterials 2018, 8, 488. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Zimbone, M.; Reitano, R.; Musumeci, P.; Calcagno, L.; Oliveri, I.P. Tyrosine capped silver nanoparticles: A new fluorescent sensor for the quantitative determination of copper(II) and cobalt(II) ions. J. Colloid Interface Sci. 2016, 462, 216–222. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Y.; Liu, H.; Wu, X.; Li, Y.; Miao, L.; Shen, Z.; Wu, A. High-performance colorimetric detection of Hg2+ based on triangular silver nanoprisms. ACS Sens. 2016, 1, 521–527. [Google Scholar] [CrossRef]
- Ghosh, S.; Maji, S.; Mondal, A. Study of selective sensing of Hg2+ ions by green synthesized silver nanoparticles suppressing the effect of Fe3+ ions. Colloids Surf. A 2018, 555, 324–331. [Google Scholar] [CrossRef]
- Zheng, W.; Liang, L.; Gu, B. Mercury reduction and oxidation by reduced natural organic matter in anoxic environments. Environ. Sci. Technol. 2012, 46, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Annadhasan, M.; Rajendiran, N. Highly selective and sensitive colorimetric detection of Hg(II) ions using green synthesized silver nanoparticles. RSC Adv. 2015, 5, 94513–94518. [Google Scholar] [CrossRef]
- Jarujamrus, P.; Amatatongchai, M.; Thima, A.; Khongrangdee, T.; Mongkontong, C. Selective colorimetric sensors based on the monitoring of an unmodified silver nanoparticles (AgNPs) reduction for a simple and rapid determination of mercury. Spectrochim. Acta Part A 2015, 142, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Stobieck, M.; Coopersmith, K.; Hepel, M. Resonance elastic light scattering (RELS) spectroscopy of fast non-Langmuirian ligand-exchange in glutathione-induced gold nanoparticle assembly. J. Colloid Interface Sci. 2010, 350, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Stobieck, M.; Hepel, M. Rapid functionalization of metal nanoparticles by moderator-tunable ligand-exchange process for biosensor designs. Sens. Actuators B Chem. 2010, 149, 373–380. [Google Scholar] [CrossRef]
- Duan, J.; Yin, H.; Wei, R.; Wang, W. Facile colorimetric detection of Hg2+ based on antiaggregation of silver nanoparticles. Biosens. Bioelectron. 2014, 57, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Yang, T.; Zhen, S.J.; Huang, C.Z. Cytosine triphosphate-capped silver nanoparticles as a platform for visual and colorimetric determination of mercury(II) and chromium(III). Microchim. Acta 2017, 184, 3171–3178. [Google Scholar] [CrossRef]
- Farhadi, K.; Forough, M.; Molaei, R.; Hajizadeh, S.; Rafipour, A. Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sens. Actuators B Chem. 2012, 161, 880–885. [Google Scholar] [CrossRef]
- Ravi, S.S.; Christena, L.R.; Subramanian, N.S.; Anthony, S.P. Green synthesized silver nanoparticles for selective colorimetric sensing of Hg2+ in aqueous solution at wide pH range. Analyst 2013, 138, 4370–4377. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.K.; Mohan, S.; Bano, D.; Gundampati, R.K.; Hasan, S.H. Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion. J. Photochem. Photobiol. B Biol. 2017, 168, 67–77. [Google Scholar] [CrossRef]
- Otto, M.; Floyd, M.; Bajpai, S. Nanotechnology for site remediation. Remediat. J. 2008, 19, 99–108. [Google Scholar] [CrossRef]
- Corsi, I.; Cherr, G.N.; Lenihan, H.S.; Labille, J.; Hasselov, M.; Canesi, L.; Dondero, F.; Frenzilli, G.; Hristozov, D.; Puntes, V.; et al. Common strategies and technologies for the ecosafety assessment and design of nanomaterials entering the marine environment. ACS Nano 2014, 8, 9694–9709. [Google Scholar] [CrossRef] [PubMed]
- Corsi, I.; Winther-Nielsen, M.; Sethi, R.; Punta, C.; Della Torre, C.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol. Environ. Saf. 2018, 154, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Dorantes-Aranda, J.J.; Waite, T.D. Silver nanoparticle–algae interactions: Oxidative dissolution, reactive oxygen species generation and synergistic toxic effects. Environ. Sci. Technol. 2012, 46, 8731–8738. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef]
- Sendra, M.; Yeste, M.; Gatica, J.; Moreno-Garrido, I.; Blasco, J. Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere 2017, 179, 279–289. [Google Scholar] [CrossRef]
- Becaro, A.A.; Jonsson, C.M.; Puti, F.C.; Siqueira, M.C.; Mattoso, L.H.; Correa, D.S.; Ferreira, M.D. Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environ. Nanotechnol. Monit. Manag. 2015, 3, 22–29. [Google Scholar] [CrossRef]
- Ale, A.; Liberatori, G.; Vannuccini, M.L.; Bergami, E.; Ancora, S.; Mariotti, G.; Bianchi, N.; Galdopórpora, J.M.; Desimone, M.F.; Cazenave, J.; et al. Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat. Toxicol. 2019, 211, 46–56. [Google Scholar] [CrossRef]
- Ribeiro, F.; Gallego-Urrea, J.A.; Jurkschat, K.; Crossley, A.; Hassellöv, M.; Taylor, C.; Soares, A.M.; Loureiro, S. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci. Total Environ. 2014, 466, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.-J.; Schwehr, K.A.; Xu, C.; Zhang, S.-J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Medei, L.; Venditti, I.; Russo, M.V.; Falconieri, M. Chemical synthesis of polyphenylacetylene nanospheres with controlled dimensions for photonic crystals. Mater. Sci. Eng. C 2003, 23, 861–865. [Google Scholar] [CrossRef]
- Venditti, I.; D’Amato, R.; Russo, M.V.; Falconieri, M. Synthesis of conjugated polymeric nanobeads for photonic bandgap materials. Sens. Actuetors B Chem. 2007, 126, 35–40. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Prairie, E., Ed.; Physical Electronics Inc.: Chanhassen, MN, USA, 1996. [Google Scholar]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 12, 4709. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High resolution XPS of organic polymers. In The Scienta ESCA 300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Secchi, V.; Krause, S.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic metal nanoparticles functionalized by 2-diethylaminoethane thiol: A close look on the metal-ligand interaction and interface chemical structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]
- Majzik, A.; Fülöp, L.; Csapó, E.; Bogár, F.; Martinek, T.; Penke, B.; Bíró, G.; Dékány, I. Functionalization of gold nanoparticles with amino acid, β-amyloid peptides and fragment. Colloids Surf. B Biointerfaces 2010, 81, 235–241. [Google Scholar] [CrossRef]
- International Organization for Standardization. Water Quality—Marine Algal Growth Inhibition Test with Skeletonema costatum and Phaeodactylum tricornutum; ISO: Geneva, Switzerland, 2006; p. 12. [Google Scholar]
- Organisation for Economic Co-operation and Development. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar] [CrossRef]
- Leal, P.P.; Hurd, C.L.; Sander, S.G.; Armstrong, E.; Roleda, M.Y. Copper ecotoxicology of marine algae: A methodological appraisal. Chem. Ecol. 2016, 32, 786–800. [Google Scholar] [CrossRef]
- Resgalla Jr, C.; Poleza, F.; Souza, R.; Máximo, M.; Radetski, C. Evaluation of effectiveness of EDTA and sodium thiosulfate in removing metal toxicity toward sea urchin embryo-larval applying the TIE. Chemosphere 2012, 89, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Bryce, D.L. Spectrometric Identification of Organic Compounds; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Frost, M.S.; Dempsey, M.J.; Whitehead, D.E. The response of citrate functionalised gold and silver nanoparticles to the addition of heavy metal ions. Colloids Surf. A 2017, 518, 15–24. [Google Scholar] [CrossRef]
- Li, S.; Liu, P.; Wang, Q. Study on the effect of surface modifier on selfaggregation behavior of Ag nanoparticle. Appl. Surf. Sci. 2012, 263, 613–618. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zou, X.; Zhang, H. Amino acid-dependent transformations of citrate-coated silver nanoparticles: Impact on morphology, stability and toxicity. Toxicol. Lett. 2014, 229, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zubavichus, Y.; Shaporenko, A.; Grunze, M.; Zharnikov, M. NEXAFS spectroscopy of biological molecules: From aminoacids to functional proteins. Nucl. Instrum. Methods Phys. Res. A 2009, 603, 111–114. [Google Scholar] [CrossRef]
- Stohr, J. NEXAFS Spectroscopy; Gomer, C., Ed.; Springer Science & Business Media: Berlin, Germany, 1991. [Google Scholar]
- National Institute of Standards and Technology. NIST X-ray Photoelectron Spectroscopy Database, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 20 September 2019).
- Gunsolus, I.L.; Mousavi, M.P.; Hussein, K.; Bühlmann, P.; Haynes, C.L. Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity. Environ. Sci. Technol. 2015, 49, 8078–8086. [Google Scholar] [CrossRef] [PubMed]
- Forough, M.; Farhadi, K. Biological and green synthesis of silver nanoparticles. Turk. J. Eng. Environ. Sci. 2010, 34, 281–287. [Google Scholar]
- Ma, Y.; Pang, Y.; Liu, F.; Xu, H.; Shen, X. Microwave-assisted ultrafast synthesis of silver nanoparticles for detection of Hg2+. Spectrochim. Acta Part A 2016, 153, 206–211. [Google Scholar] [CrossRef]
- Buduru, P.; Reddy, B.S.R.; Naidu, N.V.S. Functionalization of silver nanoparticles with glutamine and histidine for simple and selective detection of Hg2+ ion in water samples. Sens. Actuators B Chem. 2017, 244, 972–982. [Google Scholar] [CrossRef]
- Alam, A.; Ravindran, A.; Chandran, P.; Khan, S.S. Highly selective colorimetric detection and estimation of Hg2+ at nano-molar concentration by silver nanoparticles in the presence of glutathione. Spectrochim. Acta Part A 2015, 137, 503–508. [Google Scholar] [CrossRef]
- Carlini, L.; Fasolato, C.; Postorino, P.; Fratoddi, I.; Venditti, I.; Testa, G.; Battocchio, C. Comparison between silver and gold nanoparticles stabilized with negatively charged hydrophilic thiols: SR-XPS and SERS as probes for structural differences and similarities. Colloids Surf. A 2017, 532, 183–188. [Google Scholar] [CrossRef]
- Sun, T.S.; Buchner, S.P.; Byer, N.E. Oxide and interface properties of anodic films on Hg1−xCdxTe. J. Vac. Sci. Technol. 1980, 17, 1067–1073. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV–Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N. Silver nanotechnologies and the environment: Old problems or new challenges? Proj. Emerg. Nanotechnol. Rep. 2008, 15, 12–13. [Google Scholar]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef]
- Mu, L.; Gao, Y.; Hu, X. L-Cysteine: A biocompatible, breathable and beneficial coating for graphene oxide. Biomaterials 2015, 52, 301–311. [Google Scholar] [CrossRef]





| TG 201 (Freshwater) | F/2 (Marine Water) | |||
|---|---|---|---|---|
| 0 h | 72 h | 0 h | 72 h | |
| CTRL | 0.36 ± 0.06 | 0.26 ± 0.08 | 0.27 ± 0.01 | 0.26 ± 0.02 |
| AgNPs | 0.19 ± 0.02 | 0.4 ± 0.06 | 0.15 ± 0.03 | 0.37 ± 0.03 |
| AgNO3 | 4.42 ± 0.09 | 4.79 ± 0.18 | 4.37 ± 0.08 | 5.32 ± 0.22 |
| Metal NPs Functionalization | NPs Diameter (nm) | Hg2+ Detection Limit (LOD) (M) | References |
|---|---|---|---|
| Citrate/Lcysteine-AgNPs | 5 ± 2 | 3.0 × 10−6 (0.6 ppm) | This work |
| Tween 20-AuNPs | - | 1.0 × 10−7 | [8] |
| L-cys-AuNPs | - | 1.0 × 10−7 | [10] |
| Acanthe phylum bracteatum AgNPs | 29–68 | 2.2 × 10−6 | [40,74] |
| L-cysteine AgNPs | 10 | 1.0 × 10−8 | [75] |
| Glutamine/Histidine AgNPs | 5.5 ± 1.0 | 9.0 × 10−7 | [76] |
| Glutathione AgNPs | 3.9 ± 0.6 | >1.0 × 10−7 | [77] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosposito, P.; Burratti, L.; Bellingeri, A.; Protano, G.; Faleri, C.; Corsi, I.; Battocchio, C.; Iucci, G.; Tortora, L.; Secchi, V.; et al. Bifunctionalized Silver Nanoparticles as Hg2+ Plasmonic Sensor in Water: Synthesis, Characterizations, and Ecosafety. Nanomaterials 2019, 9, 1353. https://doi.org/10.3390/nano9101353
Prosposito P, Burratti L, Bellingeri A, Protano G, Faleri C, Corsi I, Battocchio C, Iucci G, Tortora L, Secchi V, et al. Bifunctionalized Silver Nanoparticles as Hg2+ Plasmonic Sensor in Water: Synthesis, Characterizations, and Ecosafety. Nanomaterials. 2019; 9(10):1353. https://doi.org/10.3390/nano9101353
Chicago/Turabian StyleProsposito, Paolo, Luca Burratti, Arianna Bellingeri, Giuseppe Protano, Claudia Faleri, Ilaria Corsi, Chiara Battocchio, Giovanna Iucci, Luca Tortora, Valeria Secchi, and et al. 2019. "Bifunctionalized Silver Nanoparticles as Hg2+ Plasmonic Sensor in Water: Synthesis, Characterizations, and Ecosafety" Nanomaterials 9, no. 10: 1353. https://doi.org/10.3390/nano9101353
APA StyleProsposito, P., Burratti, L., Bellingeri, A., Protano, G., Faleri, C., Corsi, I., Battocchio, C., Iucci, G., Tortora, L., Secchi, V., Franchi, S., & Venditti, I. (2019). Bifunctionalized Silver Nanoparticles as Hg2+ Plasmonic Sensor in Water: Synthesis, Characterizations, and Ecosafety. Nanomaterials, 9(10), 1353. https://doi.org/10.3390/nano9101353