Catalytic Effect of Facile Synthesized TiH1.971 Nanoparticles on the Hydrogen Storage Properties of MgH2
Abstract
:1. Introduction
2. Experimental
3. Results and discussion
3.1. Characterization of Nano-TiH1.971
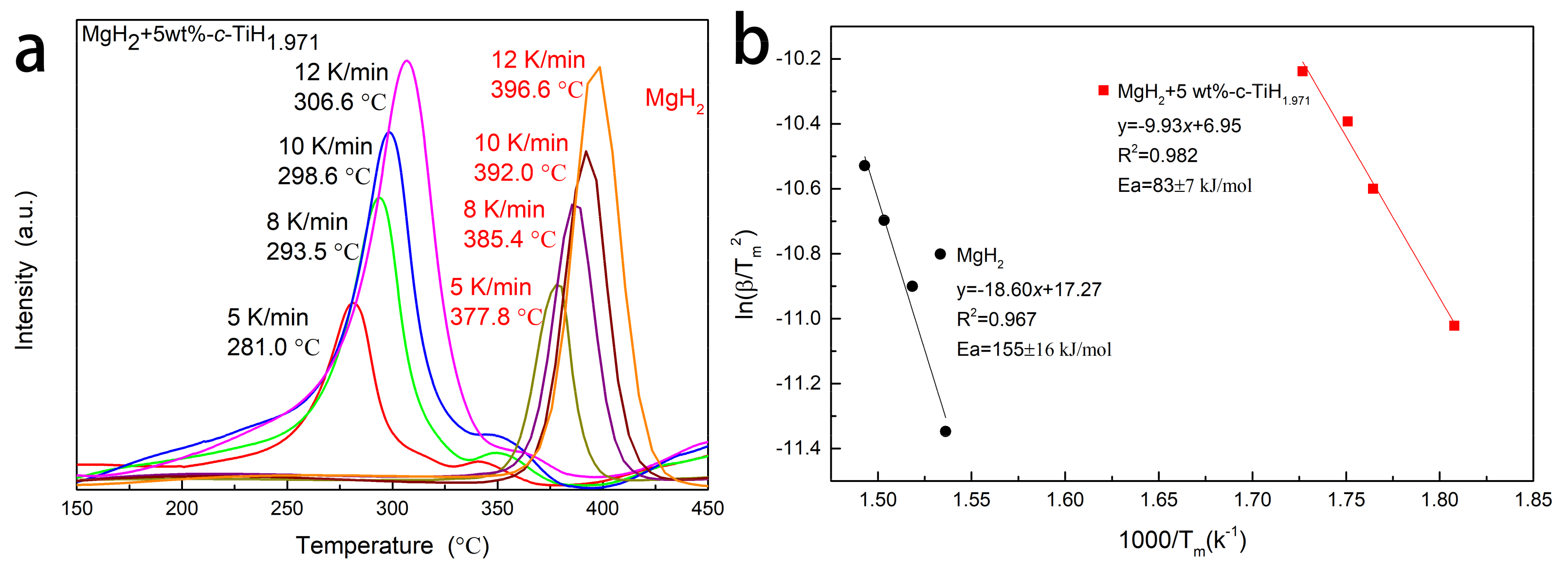
3.2. Catalytic Effect of TiH1.971 on Dehydrogenation of MgH2
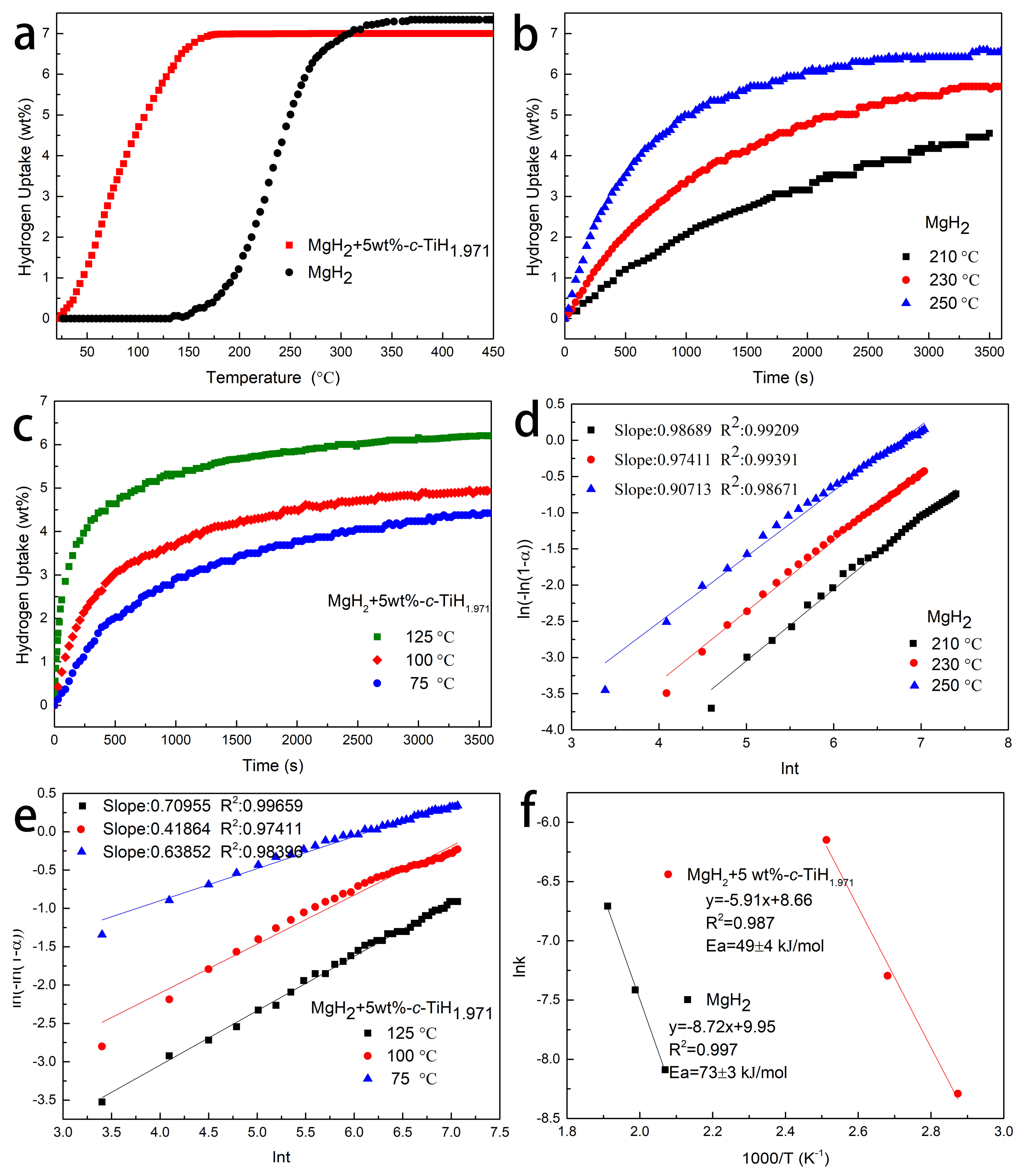
3.3. Catalytic Effect of TiH1.971 on Hydrogenation of MgH2
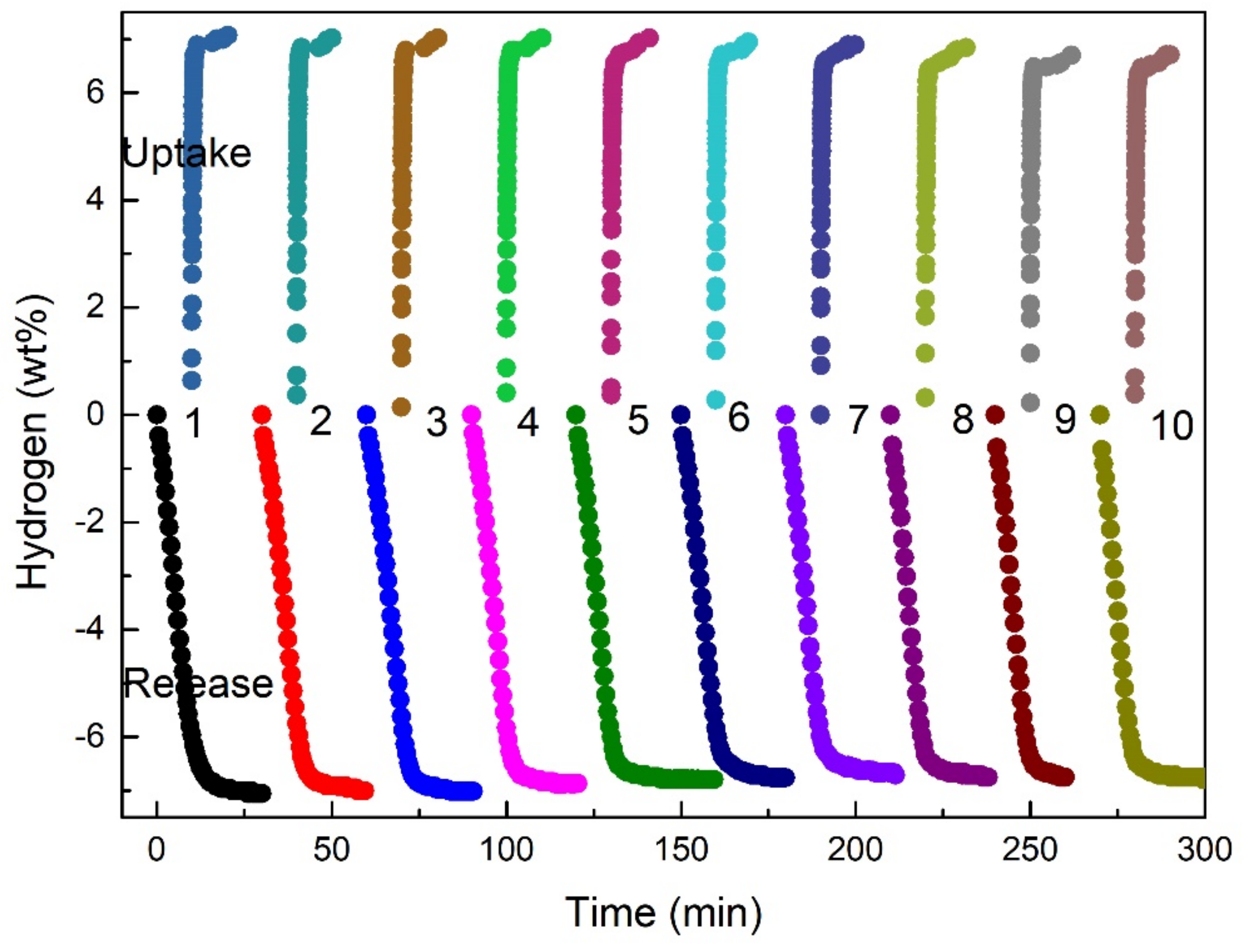
3.4. Cycling Hydrogen Storage Properties of the MgH2-TiH1.971 Composites
3.5. Evolution of TiH1.971 during Cycling and Its Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toumi, S.; Toumi, H. Asymmetric causality among renewable energy consumption, CO2 emissions, and economic growth in KSA: Evidence from a non-linear ARDL model. Environ. Sci. Pollut. Res. 2019, 26, 16145–16156. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Züttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as a Future Energy Carrier; WILEY-VCH Verlag: Weinheim, Germany, 2008. [Google Scholar]
- Schapbach, L.Z.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, Z. Effect of rGO supported NiCu derived from layered double hydroxide on hydrogen sorption kinetics of MgH2. J. Alloys Compd. 2019, 789, 768–776. [Google Scholar] [CrossRef]
- Lu, Z.; Cherepakhin, V.; Demianets, I.; Lauridsen, P.J.; Williams, T.J. Iridium-based hydride transfer catalysts: From hydrogen storage to fine chemicals. Chem. Commun. 2018, 54, 7711–7724. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Xiao, J.S.; Bnard, P.; Chahine, R. Thermal management of metal hydride hydrogen storage reservoir using phase change materials. Int. J. Hydrogen Energy 2019, 44, 21055–21066. [Google Scholar] [CrossRef]
- Zou, L.; Zhou, H.C. Hydrogen Storage in Metal-Organic Frameworks//Nanostructured Materials for Next-Generation Energy Storage and Conversion; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Khalil, Y.F. Experimental determination of dust cloud deflagration parameters of selected hydrogen storage materials: Complex metal hydrides, chemical hydrides, and adsorbents. J. Loss Prev. Process 2013, 26, 96–103. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.G.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogenation of nanocrystalline Mg at room temperature in the presence of TiH2. J. Am. Soc. 2010, 132, 6616–6617. [Google Scholar] [CrossRef]
- Morris, L.; Hales, J.J.; Trudeau, M.L. A manganese hydride molecular sieve for practical hydrogen storage under ambient conditions. Energy Environ. Sci. 2019, 12, 1580–1591. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.N.; Mao, C. Synergistic effect of Ti and F co-doping on dehydrogenation properties of MgH2 from first-principles calculations. J. Alloys Compd. 2012, 538, 205–211. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lu, J.; Sohn, H.Y. Hydrogen storage properties of the Mg–Ti–H system prepared by high-energy–high-pressure reactive milling. J. Power Sources 2008, 180, 491–497. [Google Scholar] [CrossRef]
- Tao, S.X.; Notten, P.H.L.; Van, S.R.A.; Jansen, A.P.J. Dehydrogenation properties of epitaxial (100) MgH2/TiH2 multilayers—A DFT study. Comput. Mater. Sci. 2011, 50, 2960–2966. [Google Scholar] [CrossRef]
- Imamura, H.; Masanari, K.; Kusuhara, M. High hydrogen storage capacity of nanosized magnesium synthesized by high energy ball-milling. J. Alloys Compd. 2005, 386, 211–216. [Google Scholar] [CrossRef]
- Sugai, C.; Kim, S.; Severa, G.; White, J.L.; Leick, N.; Martinez, M.B.; Gennett, T.; Stavila, V.; Jensen, C. Kinetic enhancement of direct hydrogenation of MgB2 to Mg(BH2)4 upon mechanical milling with THF, MgH2, and/or Mg. Chemphyschem 2019, 20, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Cai, Z.L.; Yao, Z.D. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Johnson, J.K.; Shaz, M.A.; Srivastava, O.N. H2 as a dynamic additive for improving the de/rehydrogenation properties of MgH2: A combined experimental and theoretical mechanistic investigation. J. Phys. Chem. C 2018, 122, 21248–21261. [Google Scholar] [CrossRef]
- Zhang, L.T.; Chen, L.X.; Fan, X.L.; Xiao, X.Z.; Zheng, J.G.; Huang, X. Enhanced hydrogen storage properties of MgH2 with numerous hydrogen diffusion channels provided by Na2Ti3O7 nanotubes. J. Mater. Chem. A 2017, 5, 6178–6185. [Google Scholar] [CrossRef]
- Shevlin, S.A.; Guo, Z.X. MgH2 dehydrogenation thermodynamics: Nanostructuring and transition metal doping. J. Phys. Chem. C 2013, 117, 10883–10891. [Google Scholar] [CrossRef]
- Florian, S.; Heiko, L.; Tobias, S. Nanoscale hydrogenography on single magnesium nanoparticles. Nano Lett. 2018, 18, 4293–4302. [Google Scholar]
- Ouyang, L.Z.; Cao, Z.J.; Wang, H. Enhanced dehydriding thermodynamics and kinetics in Mg(In)–MgF2 composite directly synthesized by plasma milling. J. Alloys Compd. 2014, 586, 113–117. [Google Scholar] [CrossRef]
- Binns, W.; Zargarzadah, F.; Dehnavi, V. Physical and electrochemical evidence for the role of a Mg hydride species in Mg alloy corrosion. Corrosion 2019, 75, 58–68. [Google Scholar] [CrossRef]
- Yong, H.; Guo, S.H.; Yuan, Z.M.; Qi, Y.; Zhao, D.L.; Zhang, Y.H. Improved hydrogen storage kinetics and thermodynamics of RE-Mg-based alloy by co-doping Ce–Y. Int. J. Hydrogen Energy 2019, 44, 16765–16776. [Google Scholar] [CrossRef]
- Praphatorn, P.; Sophida, T.; Palmarin, D. Synergistic effects of transition metal halides and activated carbon nanofibers on kinetics and reversibility of MgH2. J. Phys. Chem. Solids 2019, 124, 81–88. [Google Scholar]
- Li, L.; Tan, Y.; Zhu, Y. Excellent catalytic effects of multi-walled carbon nanotubes supported titania on hydrogen storage of Mg-Ni alloy. Chem. Commun. 2015, 51, 2368–2371. [Google Scholar]
- Alsabawi, K.; Gray, E.M.; Webb, C.J. The effect of ball-milling gas environment on the sorption kinetics of MgH2 with/without additives for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 2976–2980. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, X.; Wang, X.; Chen, M.; Lu, Y.; Liu, M.; Chen, L. Excellent catalysis of TiO2 nanosheets with high-surface-energy {001} facets on the hydrogen storage properties of MgH2. Nanoscale 2019, 11, 7465–7473. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.; Chen, J. Enhancing hydrogen storage performances of MgH2 by Ni nano-particles over mesoporous carbon CMK-3. Nanotechnology 2018, 29, 265705. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.H.; Gao, M.X.; Leng, Z.H. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2. J. Power Sources 2018, 398, 183–192. [Google Scholar]
- Kumar, S.; Tiwari, G.P. Thermodynamics and kinetics of MgH2–nfTa2O5 composite for reversible hydrogen storage application. J. Mater. Sci. 2017, 52, 6962–6968. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Jain, A. Enhancement of hydrogen desorption kinetics in magnesium hydride by doping with lithium metatitanate. J. Alloys Compd. 2017, 711, 400–405. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Lotoskyy, M.; Denys, R.; Yartys, V.A.; Eriksen, J. An outstanding effect of graphite in nano-MgH2-TiH2 on hydrogen storage performance. J. Mater. Chem. A 2018, 6, 10740–10754. [Google Scholar] [CrossRef]
- Shao, H.; Felderhoff, M.; Schüth, F. Hydrogen storage properties of nanostructured MgH2/TiH2 composite prepared by ball milling under high hydrogen pressure. Int. J. Hydrogen Energy 2011, 36, 10828–10833. [Google Scholar] [CrossRef]
- Patelli, N.; Calizzi, M.; Migliori, A. Hydrogen desorption below 150 °C in MgH2-TiH2 composite nanoparticles: Equilibrium and kinetic properties. J. Phys. Chem. C 2017, 121, 11166–11177. [Google Scholar] [CrossRef]
- Ma, X.; Xie, X.; Liu, P. Synergic catalytic effect of Ti hydride and Nb nanoparticles for improving hydrogenation and dehydrogenation kinetics of Mg-based nanocomposite. Prog. Nat. Sci. 2017, 27, 99–104. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Agarwal, S. The enhanced de/re-hydrogenation performance of MgH2 with TiH2 additive. Int. J. Energy Res. 2018, 42, 1139–1147. [Google Scholar] [CrossRef]
- Choi, Y.J.; Xu, Y.; Shaw, W.J. Hydrogen storage properties of new hydrogen-rich BH3NH3-metal hydride (TiH2, ZrH2, MgH2, and/or CaH2) composite systems. J. Phys. Chem. C 2012, 116, 8349–8358. [Google Scholar] [CrossRef]
- Zhang, L.T.; Ji, L.; Yao, Z.D.; Yan, N.H.; Sun, Z.; Yang, X.L.; Zhu, X.Q.; Hu, S.L.; Chen, L.X. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, R.; Chauhan, V. High-energy 120 MeV Au9+ ion beam-induced modifications and evaluation of craters in surface morphology of SnO2 and TiO2 nanocomposite thin films. Appl. Nanosci. 2019, 9, 1265–1280. [Google Scholar] [CrossRef]
- Luo, B.S.; Xiao, X.Z.; Zhao, S.C. Facile synthesis of Co/Pd supported by few-walled carbon nanotubes as an efficient bidirectional catalyst for improving the low temperature hydrogen storage properties of magnesium hydride. J. Mater. Chem. A 2019, 7, 5277–5287. [Google Scholar]
- Gao, M.X.; Zhang, X.; Lu, Y.H. Facile synthesis and superior catalytic activity of Nano-TiN@N-C for hydrogen storage in NaAlH4. ACS Appl. Mater. Interface 2018, 10, 15767–15777. [Google Scholar]
- Sharp, J.H.; Brindley, G.W.; Achar, B.N.N. Numerical data for some commonly used solid state reaction equations. J. Am. Ceram. Soc. 1966, 49, 379–382. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Li, H.W.; Li, Y.T. Intrinsic alterations in the hydrogen desorption of Mg2NiH4 by solid dissolution of titanium. Dalton Trans. 2018, 47, 8418–8426. [Google Scholar]
- Rougier, A.; Janot, R.; Darok, X. Hydrogen sorption properties for surface treated MgH2 and Mg2Ni alloys. J. Alloys Compd. 2005, 404, 293–296. [Google Scholar]
- Wang, Y.; Li, L.; An, C. Facile synthesis of TiN decorated graphene and its enhanced catalytic effects on dehydrogenation performance of magnesium hydride. Nanoscale 2014, 6, 6684–6691. [Google Scholar] [CrossRef]
- Idris, N.H.; Mustafa, N.S.; Ismail, M. MnFe2O4 nanopowder synthesised via a simple hydrothermal method for promoting hydrogen sorption from MgH2. Int. J. Hydrogen Energy 2017, 42, 21114–21120. [Google Scholar] [CrossRef]
- Lueking, A.; Yang, R.T. Hydrogen spillover from a metal oxide catalyst onto carbon nanotubes-implications for hydrogen storage. J. Catal. 2002, 206, 165–168. [Google Scholar] [CrossRef]
- Yang, F.H.; Yang, R.T. Ab initio molecular orbital study of adsorption of atomic hydrogen on graphite: Insight into hydrogen storage in carbon nanotubes. Carbon 2002, 40, 437–444. [Google Scholar] [CrossRef]
- Pelletier, J.F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A.R.; Lurio, L.B. Hydrogen desorption mechanism in MgH2-Nb nanocomposites. Phys. Rev. B 2002, 63, 811–820. [Google Scholar]
- Ren, C.; Fang, Z.Z.; Zhou, C.S.; Lu, J.; Ren, Y.; Zhang, X.Y.; Luo, X.Y. In situ X-ray diffraction study of dehydrogenation of MgH2 with Ti-based additives. Int. J. Hydrogen Energy 2014, 39, 5868–5873. [Google Scholar] [CrossRef]
- Yang, X.L.; Ji, L.; Yan, N.H.; Sun, Z.; Lu, X.; Zhang, L.T.; Zhu, X.Q.; Chen, L.X. Superior catalytic effects of FeCo nanosheets on MgH2 for hydrogen storage. Dalton Trans. 2019, 48, 12699–12706. [Google Scholar] [CrossRef] [PubMed]





| Symbol | Model | Integral f(α) Form |
|---|---|---|
| D1 | One-dimensional diffusion | α2 |
| D2 | Two-dimensional diffusion | α+(1-α)ln(1-α) |
| D3 | Three-dimensional diffusion(Jander equation) | [1-(1-α)1/3]2 |
| D4 | Three-dimensional diffusion(Ginstling-Braunshtein equation) | (1-2α/3)-(1-α)2/3 |
| F1 | First-order reaction | -ln(1-α) |
| R2 | Two-dimensional phase boundary | 1-(1-α)1/2 |
| R3 | Three-dimensional phase boundary | 1-(1-α)1/3 |
| A2 | Avarami-Erofe’ev | [-ln(1-α)]1/2 |
| A3 | Avarami-Erofe’ev | [-ln(1-α)]1/3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Lu, X.; Ji, L.; Yan, N.; Sun, Z.; Zhu, X. Catalytic Effect of Facile Synthesized TiH1.971 Nanoparticles on the Hydrogen Storage Properties of MgH2. Nanomaterials 2019, 9, 1370. https://doi.org/10.3390/nano9101370
Zhang L, Lu X, Ji L, Yan N, Sun Z, Zhu X. Catalytic Effect of Facile Synthesized TiH1.971 Nanoparticles on the Hydrogen Storage Properties of MgH2. Nanomaterials. 2019; 9(10):1370. https://doi.org/10.3390/nano9101370
Chicago/Turabian StyleZhang, Liuting, Xiong Lu, Liang Ji, Nianhua Yan, Ze Sun, and Xinqiao Zhu. 2019. "Catalytic Effect of Facile Synthesized TiH1.971 Nanoparticles on the Hydrogen Storage Properties of MgH2" Nanomaterials 9, no. 10: 1370. https://doi.org/10.3390/nano9101370





