Effects of Surface Functional Groups on the Adhesion of SiO2 Nanospheres to Bio-Based Materials
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Methods
2.2. Preparation of Organically Modified Silica Rose Fragrance Nanospheres
2.3. Characterization of the Morphology and Chemical Structure of Modified SiO2 Nanospheres
2.4. Application of Modified SiO2 Nanospheres on Leather
2.5. Characterization of Aromatic Leather
2.6. Interaction Between Modified SiO2 Nanospheres and Leather
3. Results and Discussion
3.1. Preparation of Modified SiO2 Nanospheres Using the Sol-Gel Method
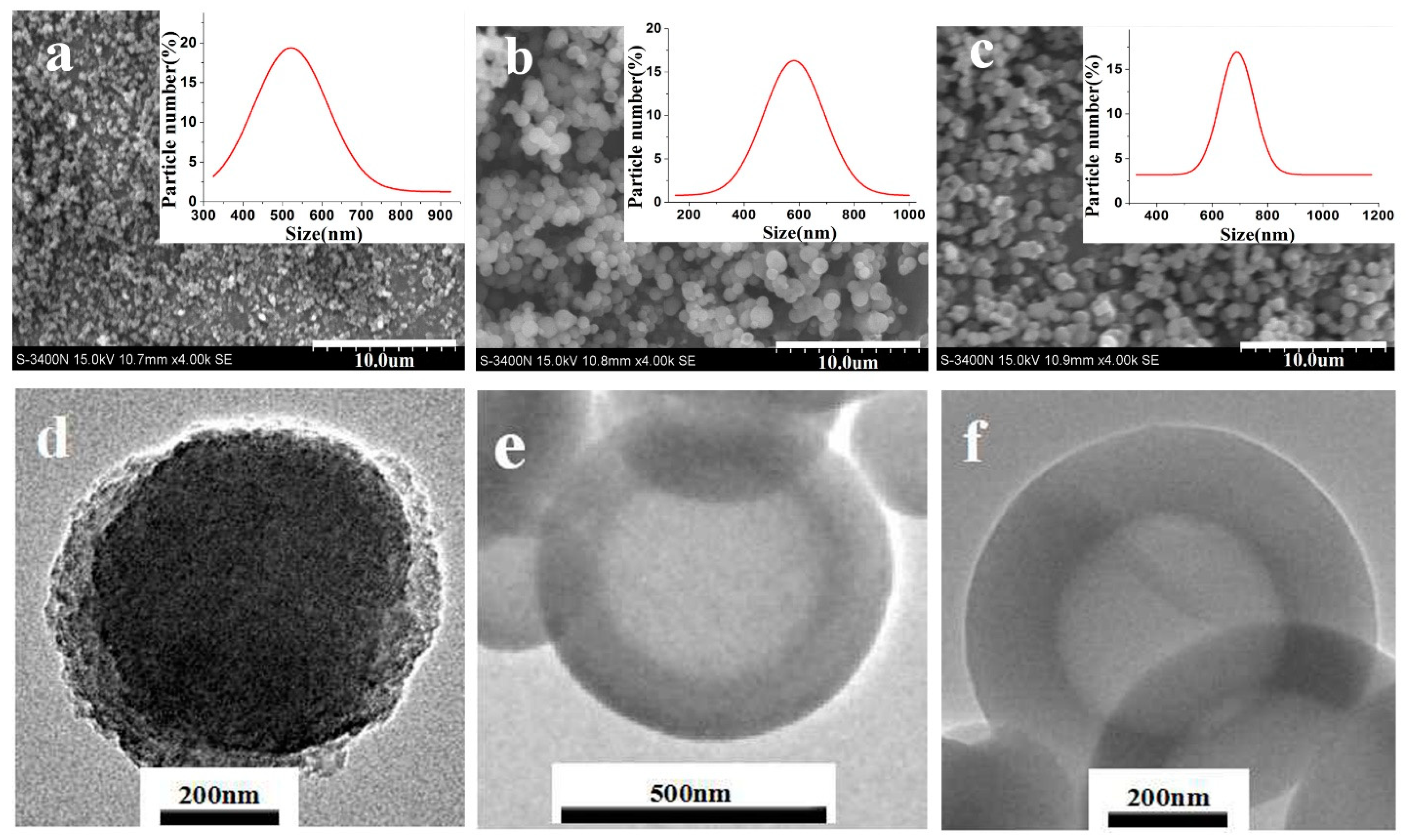
3.2. Morphology Analysis of Modified SiO2 Nanospheres
3.3. Fourier Infrared Spectrum and XRD Study of Modified SiO2 Nanospheres
3.4. Morphology Analysis of Modified SiO2 Nanospheres Leather
3.5. Thermogravimetric Analysis and Sensory Evaluation
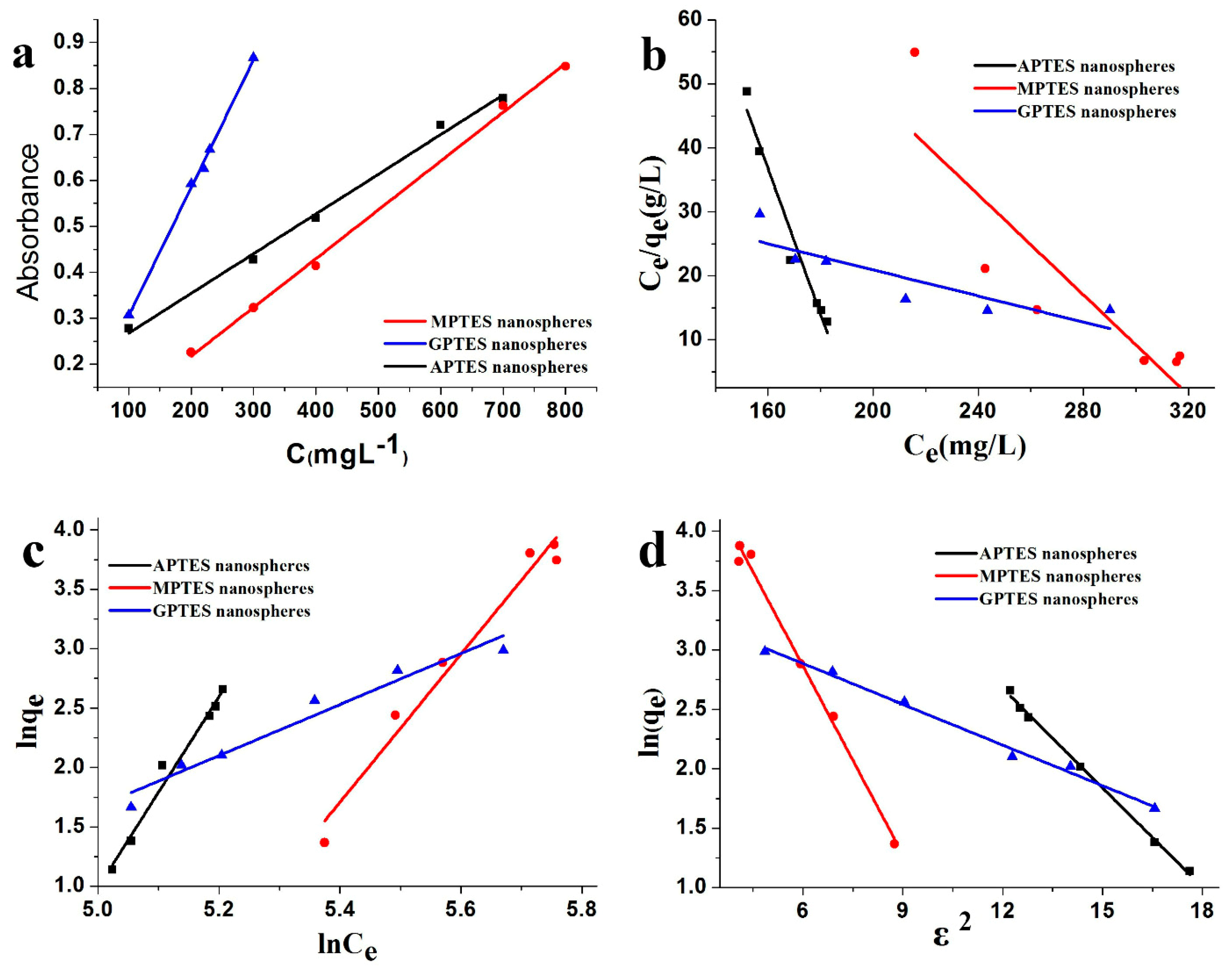
3.6. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Ye, X. Adsorption of nanoparticles on solid substrate matrix. Mater. Guide 2010, 24, 8–12. [Google Scholar]
- Gautam, R.K.; Gautam, P.K.; Banerjee, S.; Soni, S.; Singh, S.K.; Chattopadhyaya, M.C. Removal of Ni(II) by magnetic nanoparticles. J. Mol. Liq. 2015, 204, 60–69. [Google Scholar] [CrossRef]
- Tuutijarvi, T.; Lu, J.; Sillanpaa, M.; Chen, G. As(V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Huang, W.; Yu, Q.; Liu, X.; Liu, Y.; Liu, R. A rapid combustion process for the preparation of NixCu(1−x)Fe2O4 nanoparticles and their adsorption characteristics of methyl blue. Appl. Phys. A 2019, 125, 88. [Google Scholar] [CrossRef]
- Westcott, S.L.; Oldenburg, S.J.; Lee, T.R.; Halas, N.J. Formation and Adsorption of Clusters of Gold Nanoparticles onto Functionalized Silica Nanoparticle Surfaces. Langmuir ACS J. Surf. Colloids 1998, 14, 5396–5401. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Induced effect of adding nano silica on adsorption of a natural surfactant onto sandstone rock: Experimental and theoretical study. J. Pet. Sci. Eng. 2013, 112, 239–247. [Google Scholar] [CrossRef]
- Park, N.; Sung, D.; Lim, S.; Moon, S.; Hong, S. Realistic adsorption geometries and binding affinities of metal nanoparticles onto the surface of carbon nanotubes. Appl. Phys. Lett. 2009, 94, 073105. [Google Scholar] [CrossRef] [Green Version]
- Sukhov, V.M.; Dement’eva, O.V.; Kartseva, M.E.; Rudoy, V.M.; Ogarev, V.A. Metal Nanoparticles on Polymer Surfaces: 3. Adsorption Kinetics of Gold Hydrosol Particles on Polystyrene and Poly(2-vinylpyridine). Colloid J. 2004, 66, 482–488. [Google Scholar] [CrossRef]
- Cao, S.; Jin, X.; Yuan, X.; Wu, W.; Hu, J.; Sheng, W. A facile method for the preparation of monodisperse hollow silica spheres with controlled shell thickness. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1332–1338. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Jiang, Y.; Su, Q.; Zheng, J. Facile surface modification of silica nanoparticles with a combination of noncovalent and covalent methods for composites application. Compos. Sci. Technol. 2014, 104, 1–8. [Google Scholar] [CrossRef]
- Si, Y.; Guo, Z. Superwetting Materials of Oil–Water Emulsion Separation. Chem. Lett. 2015, 44, 874–883. [Google Scholar] [CrossRef]
- Park, J.Y.; Back, S.H.; Chang, S.J.; Lee, S.J.; Lee, K.G.; Park, T.J. Dopamine-assisted synthesis of carbon-coated silica for PCR enhancement. ACS Appl. Mater. Interfaces 2015, 7, 15633–15640. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, Y.; Wu, L.; You, B.; Zi, J. Fabrication of robust crystal balls from the electrospray of soft polymer spheres/silica dispersion. Langmuir ACS J. Surf. Colloids 2010, 26, 6604–6609. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, A.; Sun, J.; Wang, L. Properties of Natural Rubber Vulcanizates/Nanosilica Composites Prepared Based on the Method of In-situ Generation and Coagulation. J. Macromol. Sci. Part B 2013, 52, 1494–1507. [Google Scholar] [CrossRef]
- Yin, J.; Deng, T.; Zhang, G. Preparation and size control of highly monodisperse vinyl functionalized silica spheres. Appl. Surf. Sci. 2012, 258, 1910–1914. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Q.; Li, H.; Hu, Y. Polyethylene-Modified Nano Silica and Its Fine Dispersion in Polyethylene. Ind. Eng. Chem. Res. 2017, 56, 5892–5898. [Google Scholar] [CrossRef]
- Singh, R.; Bapat, R.; Qin, L.; Feng, H.; Polshettiwar, V. Atomic Layer Deposited (ALD) TiO2 on Fibrous Nano-Silica (KCC-1) for Photocatalysis: Nanoparticle Formation and Size Quantization Effect. ACS Catal. 2016, 6, 2770–2784. [Google Scholar] [CrossRef]
- Ramalingam, S.; Sreeram, K.J.; Raghava Rao, J.; Unni Nair, B. Organic Nanocolorants: Self-Fixed, Optothermal Resistive, Silica-Supported Dyes for Sustainable Dyeing of Leather. ACS Sustain. Chem. Eng. 2016, 4, 2706–2714. [Google Scholar] [CrossRef]
- Fan, Q.; Ma, J.; Xu, Q.; Wang, J.; Ma, Y. Facile Synthesis of Chitosan-Coated Silica Nanocapsules via Interfacial Condensation Approach for Sustained Release of Vanillin. Ind. Eng. Chem. Res. 2018, 57, 6171–6179. [Google Scholar] [CrossRef]
- Ma, J.; Hu, J.; Yang, Z. Preparation of Acrylic Resin/Nano-SiO2for Leather Finishing Agent. Mater. Manuf. Process. 2007, 22, 782–786. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Niu, Y.; Zhu, G.; Deng, J.; Liu, S. Lavender fragrance sol-gel encapsulated in ORMOSIL nanospheres. Flavour Fragr. J. 2019, 34, 21–27. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, S.; Chen, K.; Gao, X.; Chang, P.; Tian, C.; Wang, J.; Huang, Y. Preparation and properties of nanoencapsulated n-octadecane phase change material with organosilica shell for thermal energy storage. Energy Convers. Manag. 2015, 105, 908–917. [Google Scholar] [CrossRef]
- Wang, M.; Fu, H.; She, Y.; Xiao, Z.; Zhu, G.; Hu, J. Adsorption capacity, kinetics, and thermodynamics of chitosan nanoparticles onto cotton fabrics without any chemical binders. Polym. Compos. 2015, 36, 2093–2102. [Google Scholar] [CrossRef]
- Chien-To Hesieh, H.T. Langmuir and Dubinin–Radushkevich analyses on equilibrium adsorption of activated carbon fabrics in aqueous solutions. J. Chem. Technol. Biotechnol. 2000, 1066–1072. [Google Scholar] [CrossRef]
- Visa, M.; Chelaru, A.-M. Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl. Surf. Sci. 2014, 303, 14–22. [Google Scholar] [CrossRef]
- Yang, R.; Hu, T.D.; Liu, T.; Xiang, H. Characterization of CuO-BaO/SiO2 Catalysts structure. Acta Phys. -Chim. Sin. 1998, 14, 590–595. [Google Scholar]
- Fathima, N.N.; Dhathathreyan, A.; Ramasami, T. Mercury Intrusion Porosimetry, Nitrogen Adsorption, and Scanning Electron Microscopy Analysis of Pores in Skin. Biomacromolecules 2002, 3, 899–904. [Google Scholar] [CrossRef]
- Wang, S.; Ye, L.; Liu, Y.; Zhu, Z.; Liu, J.; Xiao, Z.; Shen, Y.; Jiang, L. Fibrous pore structure of silk fabric, cattle leather and wallpaper base paper and their adsorption properties. Sci. Sin. Chim. 2019, 49, 619–624. [Google Scholar] [CrossRef]
- Gong, J.; Dan, W.; Dan, N. Study on the modification of decellularized porcine dermal matrix with quercetin. Leather Sci. Eng. 2018, 28. [Google Scholar]
- Hui, P.; Zhijun, Z.; Juxian, Z.; Zhishen, W.; Hongxin, D. Preparation and application of a new nanocomposite tanning agent MPNS/SMA. China Leather 2004, 33, 2. [Google Scholar]
- Liu, D.; Hao, L.; Fang, K. Adsorption of cationic copolymer nanospheres onto cotton fibers investigated by a facile nephelometry. Colloids Surf. A Physicochem. Eng. Asp. 2014, 452, 82–88. [Google Scholar] [CrossRef]
- Onyango, M.S.; Kojima, Y.; Aoyi, O.; Bernardo, E.C.; Matsuda, H. Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J. Colloid Interface Sci. 2004, 279, 341–350. [Google Scholar] [CrossRef] [PubMed]




| Agent | Chemical Construction | Relative Molecular Mass |
|---|---|---|
| TEOS | 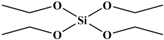 | 208 |
| APTES | 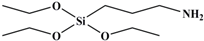 | 221 |
| MPTES | 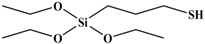 | 238 |
| GPTES |  | 278 |
| Silane Coupling Agent | Mass Before Adsorption (g) | Mass After Adsorption (g) | Δ (g) | Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| APTES | 2.6846 | 2.8165 | 0.1319 | 26.5760 |
| MPTES | 2.8559 | 2.9800 | 0.1241 | 15.3520 |
| GPTES | 2.9367 | 3.1000 | 0.1633 | 34.2577 |
| Evaluator | APTES-Modified SiO2 Nanospheres Leather | MPTES-Modified SiO2 Nanospheres Leather | GPTES-Modified SiO2 Nanospheres Leather |
|---|---|---|---|
| 1 | 7.0 | 5.0 | 9.0 |
| 2 | 6.0 | 5.0 | 9.0 |
| 3 | 8.0 | 6.0 | 8.0 |
| 4 | 9.0 | 7.0 | 9.0 |
| 5 | 8.0 | 4.0 | 9.0 |
| 6 | 9.0 | 6.0 | 8.0 |
| 7 | 8.0 | 7.0 | 9.0 |
| 8 | 7.0 | 6.0 | 8.0 |
| 9 | 8.0 | 7.0 | 9.0 |
| 10 | 8.0 | 6.0 | 9.0 |
| Average score | 7.8 | 5.9 | 8.7 |
| Nanospheres | KD (mol2kJ−2) | E (kJ/mol) | Beer–Lambert Standard Curve R2 | Dubinin–Radushkevich Adsorption Isotherm R2 |
|---|---|---|---|---|
| APTES | 0.27839 | 1.34016 | 0.99340 | 0.99710 |
| MPTES | 0.52825 | 0.97289 | 0.99757 | 0.99023 |
| GPTES | 0.11411 | 2.09326 | 0.99340 | 0.99083 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Xu, J.; Niu, Y.; Zhu, G.; Kou, X. Effects of Surface Functional Groups on the Adhesion of SiO2 Nanospheres to Bio-Based Materials. Nanomaterials 2019, 9, 1411. https://doi.org/10.3390/nano9101411
Xiao Z, Xu J, Niu Y, Zhu G, Kou X. Effects of Surface Functional Groups on the Adhesion of SiO2 Nanospheres to Bio-Based Materials. Nanomaterials. 2019; 9(10):1411. https://doi.org/10.3390/nano9101411
Chicago/Turabian StyleXiao, Zuobing, Jing Xu, Yunwei Niu, Guangyong Zhu, and Xingran Kou. 2019. "Effects of Surface Functional Groups on the Adhesion of SiO2 Nanospheres to Bio-Based Materials" Nanomaterials 9, no. 10: 1411. https://doi.org/10.3390/nano9101411





