An Integrated Structural Air Electrode Based on Parallel Porous Nitrogen-Doped Carbon Nanotube Arrays for Rechargeable Li–Air Batteries
Abstract
:1. Introduction
2. Experimental
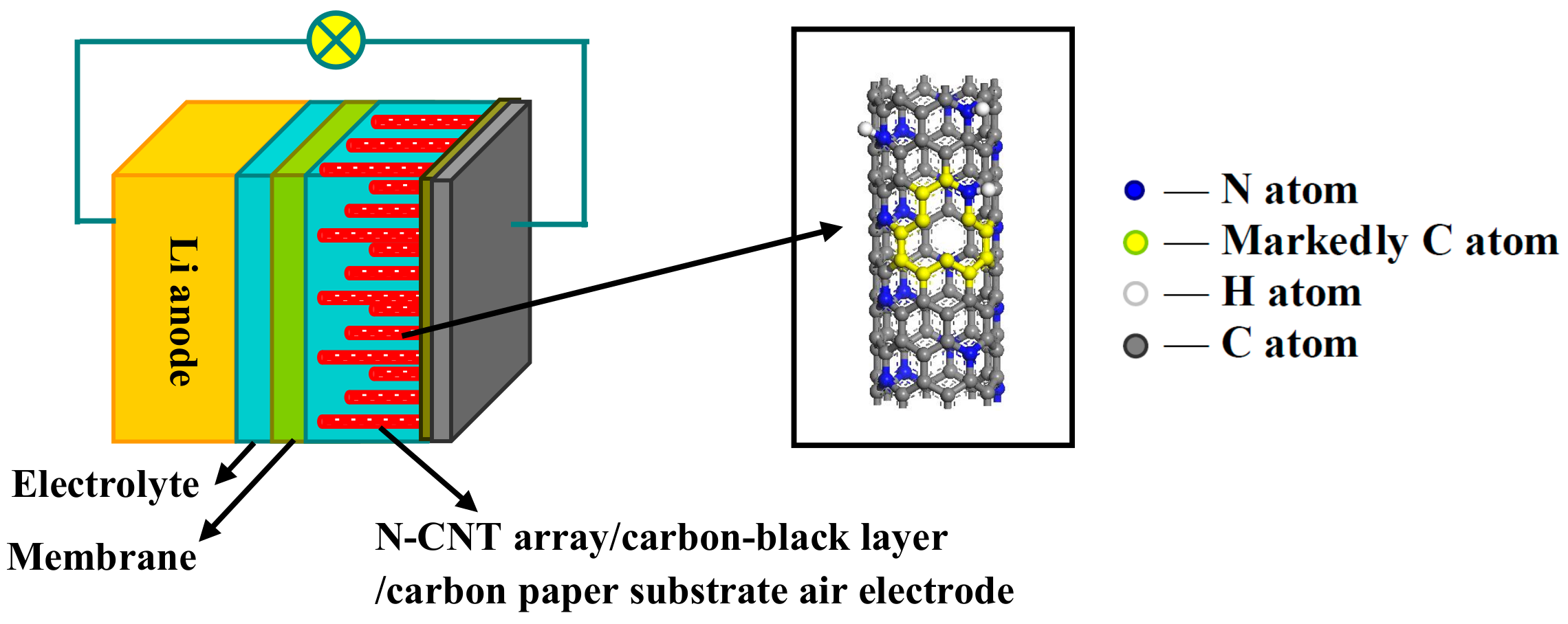
2.1. Preparation of N-CNT Arrays on a Carbon-Black Layer/Carbon Paper Substrate
2.2. Characterizations of N-CNT Arrays
2.3. Battery Assembly and Electrochemical Performance Tests
3. Results
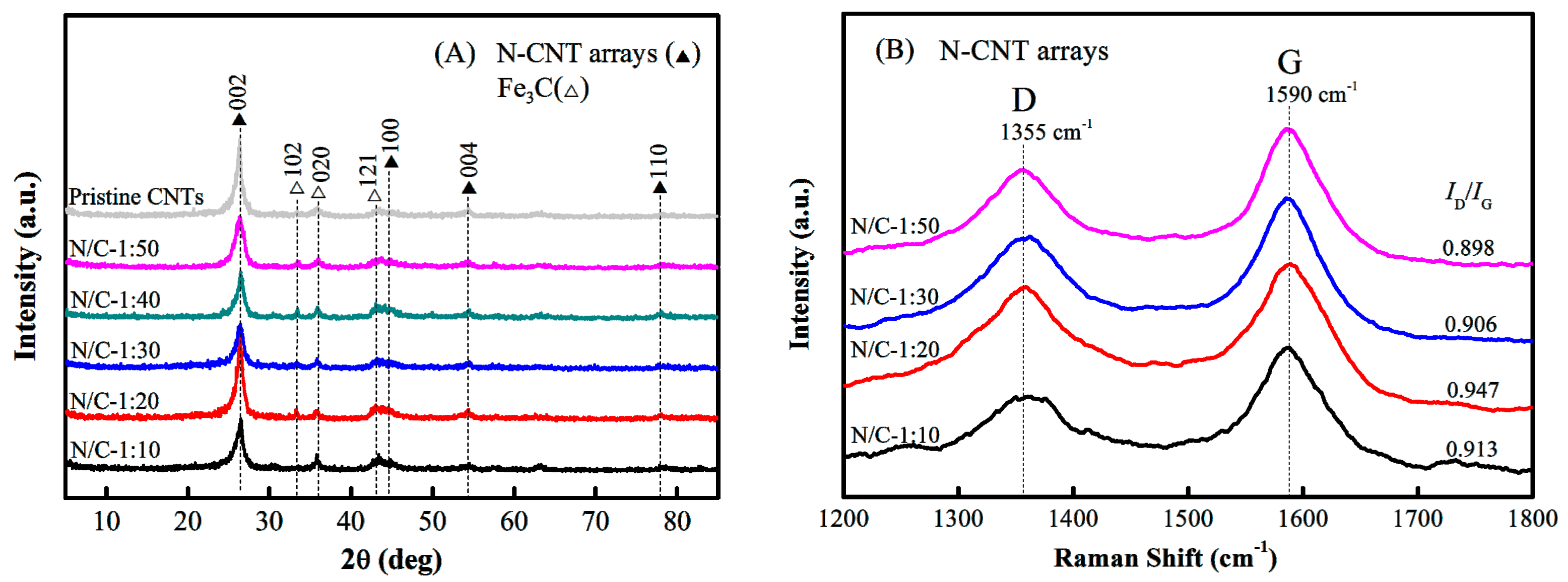
3.1. Morphology and Structure of the N-CNT Arrays
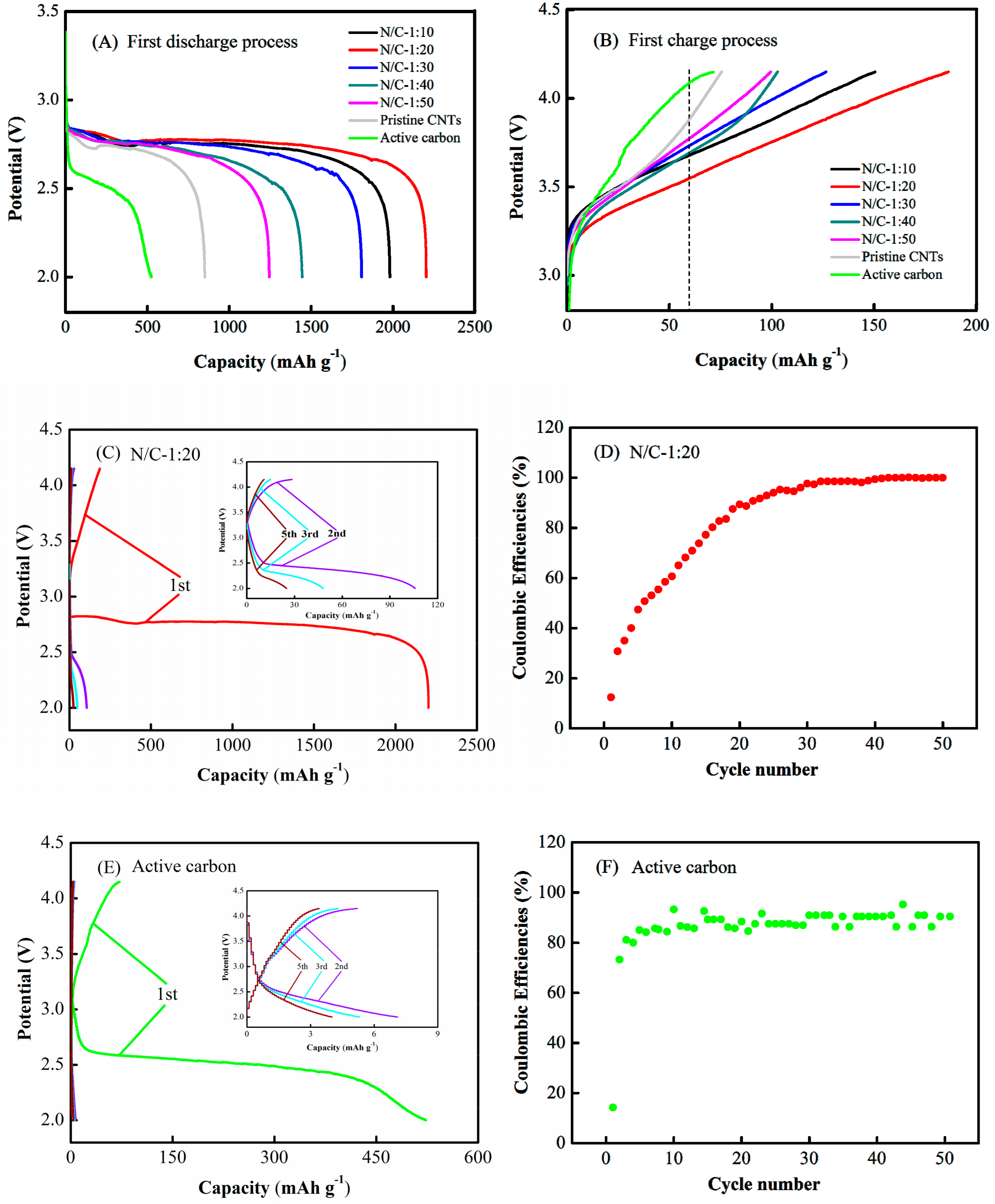
3.2. Electrochemical Performance of Li–Air Batteries
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.S.; Tai Kim, S.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design principles for heteroatom-doped carbon nanomaterials as highly efficient catalysts for fuel cells and metal–air batteries. Adv. Mater. 2015, 27, 6834–6840. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, F.; Xiao, J.; Zhang, J.; Xu, W.; Park, S.; Zhang, J.G.; Wang, Y.; Liu, J. Making Li–air batteries rechargeable: Material challenges. Adv. Funct. Mater. 2012, 23, 987–1004. [Google Scholar] [CrossRef]
- Li, F.; Zhang, T.; Zhou, H. Challenges of non-aqueous Li-O2 batteries: Electrolytes, catalysts, and anodes. Energy Environ. Sci. 2013, 6, 1125–1141. [Google Scholar] [CrossRef]
- Imanishi, N.; Hasegawa, S.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Lithium anode for lithium-air secondary batteries. J. Power Sources 2008, 185, 1392–1397. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, D.; Xu, W.; Xiao, J.; Williford, R.E. Ambient operation of Li/air batteries. J. Power Sources 2010, 195, 4332–4337. [Google Scholar] [CrossRef]
- Balaish, M.; Kraytsberg, A.; Ein-Eli, Y. A critical review on lithium-air battery electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 2801–2822. [Google Scholar] [CrossRef]
- He, P.; Zhang, T.; Jiang, J.; Zhou, H. Lithium-air batteries with hybrid electrolytes. J. Phys. Chem. Lett. 2016, 7, 1267–1280. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, H. From Li–O2 to Li–air batteries: Carbon nanotubes/ionic liquid gels with a tricontinuous passage of electrons, ions, and oxygen. Angew. Chem. Int. Ed. 2012, 51, 11062–11067. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, J.; He, C.; Chen, Y.; Wei, J.; Zhou, Z. Hierarchical carbon-nitrogen architectures with both mesopores and macrochannels as excellent cathodes for rechargeable Li-O2 batteries. Adv. Funct. Mater. 2014, 24, 6826–6833. [Google Scholar] [CrossRef]
- Xie, J.; Yao, X.; Cheng, Q.; Madden, I.P.; Dornath, P.; Chang, C.C.; Fan, W.; Wang, D. Three dimensionally ordered mesoporous carbon as a stable, high-performance Li-O2 battery cathode. Angew. Chem. Int. Ed. 2015, 54, 4299–4303. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Waletzko, M.; Chen, L.; Peppler, K.; Klar, P.J.; Janek, J. Evolution of Li2O2 growth and its effect on kinetics of Li-O2 batteries. ACS Appl. Mater. Interfaces 2014, 6, 12083–12092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, C.; Liu, S.; Yang, J.; Fan, X.; Huang, H.; Qiu, J. 3D porous N-doped graphene frameworks made of interconnected nanocages for ultrahigh-rate and long-life Li-O2 batteries. Adv. Funct. Mater. 2015, 25, 6913–6920. [Google Scholar] [CrossRef]
- Zhang, T.; Imanishi, N.; Shimonishi, Y.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A novel high energy density rechargeable lithium/air battery. Chem. Commun. 2010, 46, 1661–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.G.; Hassoun, J.; Park, J.B.; Sun, Y.K.; Scrosati, B. An improved high-performance lithium-air battery. Nat. Chem. 2012, 4, 579–585. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Fellner, J.P.; Brutchen, G.W. Diffusion-limited model for a lithium/air battery with an organic electrolyte. J. Power Sources 2007, 164, 365–371. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, J.S.; Sundén, B. Review on mechanisms and continuum models of multi-phase transport phenomena in porous structures of non-aqueous Li-air batteries. J. Power Sources 2015, 278, 352–369. [Google Scholar] [CrossRef]
- Hine, F. Electrode Processes and Electrochemical Engineering; Plenum Press: New York, NY, USA, 1985; pp. 235–252. [Google Scholar]
- Tran, C.; Yang, X.Q.; Qu, D. Investigation of the gas-diffusion-electrode used as lithium/air cathode in non-aqueous electrolyte and the importance of carbon material porosity. J. Power Sources 2010, 195, 2057–2063. [Google Scholar] [CrossRef]
- Sergeev, A.V.; Chertovich, A.V.; Itkis, D.M.; Goodilin, E.A.; Khokhlov, A.R. Effects of cathode and electrolyte properties on lithium-air battery performance: Computational study. J. Power Sources 2015, 279, 707–712. [Google Scholar] [CrossRef]
- Luo, W.B.; Gao, X.W.; Shi, D.Q.; Chou, S.L.; Wang, J.Z.; Liu, H.K. Binder-free and carbon-free 3D porous air electrode for Li-O2 batteries with high efficiency, high capacity, and long life. Small 2016, 12, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Shen, C.; Lu, Y. Air electrode for the lithium-air batteries: Materials and structure designs. ChemPlusChem 2015, 80, 270–287. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, X.; Li, L.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Zhang, J. A review of cathode materials and structures for rechargeable lithium-air batteries. Energy Environ. Sci. 2015, 8, 2144–2198. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zhang, Z.; Duan, D.; Hao, X.; Liu, S. Preparation and structural evolution of well aligned-carbon nanotube arrays onto conductive carbon-black layer/carbon paper substrate with enhanced discharge capacity for Li-air batteries. Chem. Eng. J. 2016, 283, 911–921. [Google Scholar] [CrossRef]
- Yu, R.; Fan, W.; Guo, X.; Dong, S. Highly ordered and ultra-long carbon nanotube arrays as air cathodes for high-energy-efficiency Li-oxygen batteries. J. Power Sources 2016, 306, 402–407. [Google Scholar] [CrossRef]
- Li, L.; Xing, Y. Electrochemical durability of carbon nanotubes in noncatalyzed and catalyzed oxidations. J. Electrochem. Soc. 2006, 153, A1823–A1828. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Fang, Y.; Li, X.; Zhao, S.; Wu, J.; Li, F.; Tian, M.; Long, X.; Jin, J.; Ma, J. Coaxial ultrathin Co1-yFeyOx nanosheet coating on carbon nanotubes for water oxidation with excellent activity. RSC Adv. 2016, 6, 80613–80620. [Google Scholar] [CrossRef]
- Ji, D.; Peng, S.; Safanama, D.; Yu, H.; Li, L.; Yang, G.; Qin, X.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Design of 3-dimensional hierarchical architectures of carbon and highly active transition metals (Fe, Co, Ni) as bifunctional oxygen catalysts for hybrid lithium-air batteries. Chem. Mater. 2017, 29, 1665–1675. [Google Scholar] [CrossRef]
- Das, D.; Plazas-Tuttle, J.; Sabaraya, I.V.; Jain, S.S.; Sabo-Attwood, T.; Saleh, N.B. An elegant method for large scale synthesis of metal oxide-carbon nanotube nanohybrids for nano-environmental application and implication studies. Environ. Sci. Nano 2017, 4, 60–68. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, X.; Liu, J.; Geng, D.; Yang, J.; Li, R.; Sun, X. Nitrogen-doped carbon nanotubes as cathode for lithium-air batteries. Electrochem. Commun. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Huang, K.; Carnahan, D.; Xing, Y. Hybrid Li-air battery cathodes with sparse carbon nanotube arrays directly grown on carbon fiber papers. Energy Environ. Sci. 2013, 6, 3339–3345. [Google Scholar] [CrossRef]
- Mi, R.; Liu, H.; Wang, H.; Wong, K.W.; Mei, J.; Chen, Y.; Lau, W.M.; Yan, H. Effects of nitrogen-doped carbon nanotubes on the discharge performance of Li-air batteries. Carbon 2014, 67, 744–752. [Google Scholar] [CrossRef]
- Kang, J.; Kim, D.Y.; Suk, J.; Lee, S.S.; Kim, D.W.; Kim, J.; Kang, Y. Enhanced energy and O2 evolution efficiency using an in situ electrochemically N-doped carbon electrode in non-aqueous Li-O2 batteries. J. Mater. Chem. A 2015, 3, 18843–18846. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Désilets, S.; Abou-Rachid, H.; Jaidann, M.; Lussier, L.S. Structural and morphological control of aligned nitrogen-doped carbon nanotubes. Carbon 2010, 48, 1498–1507. [Google Scholar] [CrossRef]
- Boncel, S.; Pattinson, S.W.; Geiser, V.; Shaffer, M.S.P.; Koziol, K.K.K. En route to controlled catalytic CVD synthesis of densely packed and vertically aligned nitrogen-doped carbon nanotube arrays. Beilstein J. Nanotechnol. 2014, 5, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Lyubutin, I.S.; Anosova, O.A.; Frolov, K.V.; Sulyanov, S.N.; Okotrub, A.V.; Kudashov, A.G.; Bulusheva, L.G. Iron nanoparticles in aligned arrays of pure and nitrogen-doped carbon nanotubes. Carbon 2012, 50, 2628–2634. [Google Scholar] [CrossRef]
- Mo, Z.; Liao, S.; Zheng, Y.; Fu, Z. Preparation of nitrogen-doped carbon nanotube arrays and their catalysis towards cathodic oxygen reduction in acidic and alkaline media. Carbon 2012, 50, 2620–2627. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Yin, J.; Qiu, Y.; Yu, J.; Zhou, X.; Wu, W. Enhancement of electrocatalytic activity for oxygen reduction reaction in alkaline and acid media from electrospun nitrogen-doped carbon nanofibers by surface modification. RSC Adv. 2013, 3, 15655–15663. [Google Scholar] [CrossRef]
- Gittleson, F.S.; Yao, K.P.C.; Kwabi, D.G.; Sayed, S.Y.; Ryu, W.H.; Shao-Horn, Y.; Taylor, A.D. Raman spectroscopy in lithium-oxygen battery systems. ChemElectroChem 2015, 2, 1446–1457. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Kurenya, A.G.; Zhang, H.; Zhang, H.; Chen, X.; Song, H. Electrochemical properties of nitrogen-doped carbon nanotube anode in Li-ion batteries. Carbon 2011, 49, 4013–4023. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, T.; Zhou, Y.; Li, Y.; Lu, J.; Tian, X.; Wang, D.; Wang, J. Facile synthesis of boron and nitrogen-doped graphene as efficient electrocatalyst for the oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energ. 2014, 39, 16043–16052. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Chen, Z. Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 2010, 48, 3057–3065. [Google Scholar] [CrossRef]
- Feng, L.; Yan, Y.; Chen, Y.; Wang, L. Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1892–1899. [Google Scholar] [CrossRef]
- Wang, J.Z.; Lu, L.; Lotya, M.; Coleman, J.N.; Chou, S.L.; Liu, H.K.; Minett, A.I.; Chen, J. Development of MoS2-CNT composite thin film from layered MoS2 for lithium batteries. Adv. Energy Mater. 2013, 3, 798–805. [Google Scholar] [CrossRef]
- Højberg, J.; McCloskey, B.D.; Hjelm, J.; Vegge, T.; Johansen, K.; Norby, P.; Luntz, A.C. An electrochemical impedance spectroscopy investigation of the overpotentials in Li-O2 batteries. ACS Appl. Mater. Interfaces 2015, 7, 4039–4047. [Google Scholar] [CrossRef]
- Uludağ, A.A.; Tokur, M.; Algul, H.; Cetinkaya, T.; Uysal, M.; Akbulut, H. High stable Li-air battery cells by using PEO and PVDF additives in the TEGDME/LiPF6 electrolytes. Int. J. Hydrogen Energ. 2016, 41, 6954–6964. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium-air and sodium-sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.; Cruickshank, J. Performance and modelling of a direct methanol solid polymer electrolyte fuel cell. J. Power Sources 1997, 65, 159–171. [Google Scholar] [CrossRef]
- Sahapatsombut, U.; Cheng, H.; Scott, K. Modelling the micro-macro homogeneous cycling behaviour of a lithium-air battery. J. Power Sources 2013, 227, 243–253. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Duan, D.; Sun, Y.; Wei, G.; Hao, X.; Liu, S.; Han, Y.; Meng, W. The correlation of the properties of pyrrolidinium-based ionic liquid electrolytes with the discharge-charge performances of rechargeable Li-O2 batteries. J. Power Sources 2016, 329, 207–215. [Google Scholar] [CrossRef]
- Read, J.; Mutolo, K.; Ervin, M.; Behl, W.; Wolfenstine, J.; Driedger, A.; Foster, D. Oxygen transport properties of organic electrolytes and performance of lithium/oxygen battery. J. Electrochem. Soc. 2003, 150, A1351–A1356. [Google Scholar] [CrossRef]
- Wang, Y. Modeling discharge deposit formation and its effect on lithium-air battery performance. Electrochim. Acta 2012, 75, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Luntz, A.C.; Viswanathan, V.; Voss, J.; Varley, J.B.; Nørskov, J.K.; Scheffler, R.; Speidel, A. Tunneling and polaron charge transport through Li2O2 in Li-O2 batteries. J. Phys. Chem. Lett. 2013, 4, 3494–3499. [Google Scholar] [CrossRef]
- Gallant, B.M.; Kwabi, D.G.; Mitchell, R.R.; Zhou, J.; Thompson, C.V.; Shao-Horn, Y. Influence of Li2O2 morphology on oxygen reduction and evolution kinetics in Li-O2 batteries. Energy Environ. Sci. 2013, 6, 2518–2528. [Google Scholar] [CrossRef]
- Varley, J.B.; Viswanathan, V.; Nørskov, J.K.; Luntz, A.C. Lithium and oxygen vacancies and their role in Li2O2 charge transport in Li-O2 batteries. Energy Environ. Sci. 2014, 7, 720–727. [Google Scholar] [CrossRef]






| Sample | RΩ (Ω) | Rct (Ω) | Zw (Ω) |
|---|---|---|---|
| N-CNTs (N/C = 1:10) | 16.3 | 57.9 | 32.9 |
| N-CNTs (N/C = 1:20) | 15.3 | 39.7 | 27.7 |
| N-CNTs (N/C = 1:30) | 36.6 | 157.1 | 34.5 |
| N-CNTs (N/C = 1:40) | 61.3 | 240.9 | 86.1 |
| N-CNTs (N/C = 1:50) | 58.3 | 320.2 | 60.2 |
| Pristine CNTs | 52.2 | 443.6 | 75.1 |
| Active carbon | 61.5 | 373.0 | 189.8 |
| Cycle | RΩ (Ω) | Rct (Ω) | Zw (Ω) |
|---|---|---|---|
| After 1st cycling | 248.4 | 239.9 | 404.3 |
| After 2nd cycling | 265.6 | 895.9 | 665.1 |
| After 3rd cycling | 273.9 | 1723.7 | 1651.6 |
| After 4th cycling | 276.7 | 2125.9 | 2691.3 |
| After 5th cycling | 466.7 | 2483.7 | 2978.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Z.; Duan, D.; Han, Y.; Wang, K.; Hao, X.; Wang, J.; Liu, S.; Wu, F. An Integrated Structural Air Electrode Based on Parallel Porous Nitrogen-Doped Carbon Nanotube Arrays for Rechargeable Li–Air Batteries. Nanomaterials 2019, 9, 1412. https://doi.org/10.3390/nano9101412
Li Y, Zhang Z, Duan D, Han Y, Wang K, Hao X, Wang J, Liu S, Wu F. An Integrated Structural Air Electrode Based on Parallel Porous Nitrogen-Doped Carbon Nanotube Arrays for Rechargeable Li–Air Batteries. Nanomaterials. 2019; 9(10):1412. https://doi.org/10.3390/nano9101412
Chicago/Turabian StyleLi, Yu, Zhonglin Zhang, Donghong Duan, Yunxia Han, Kunlei Wang, Xiaogang Hao, Junwen Wang, Shibin Liu, and Fanhua Wu. 2019. "An Integrated Structural Air Electrode Based on Parallel Porous Nitrogen-Doped Carbon Nanotube Arrays for Rechargeable Li–Air Batteries" Nanomaterials 9, no. 10: 1412. https://doi.org/10.3390/nano9101412





