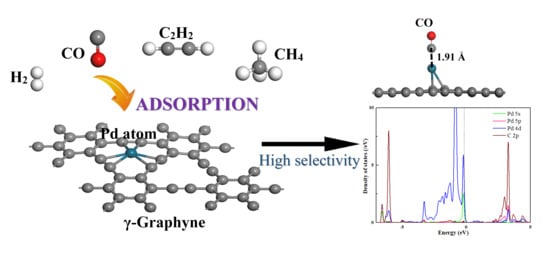
Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation
Abstract
1. Introduction
2. Methods
3. Results and Discussion
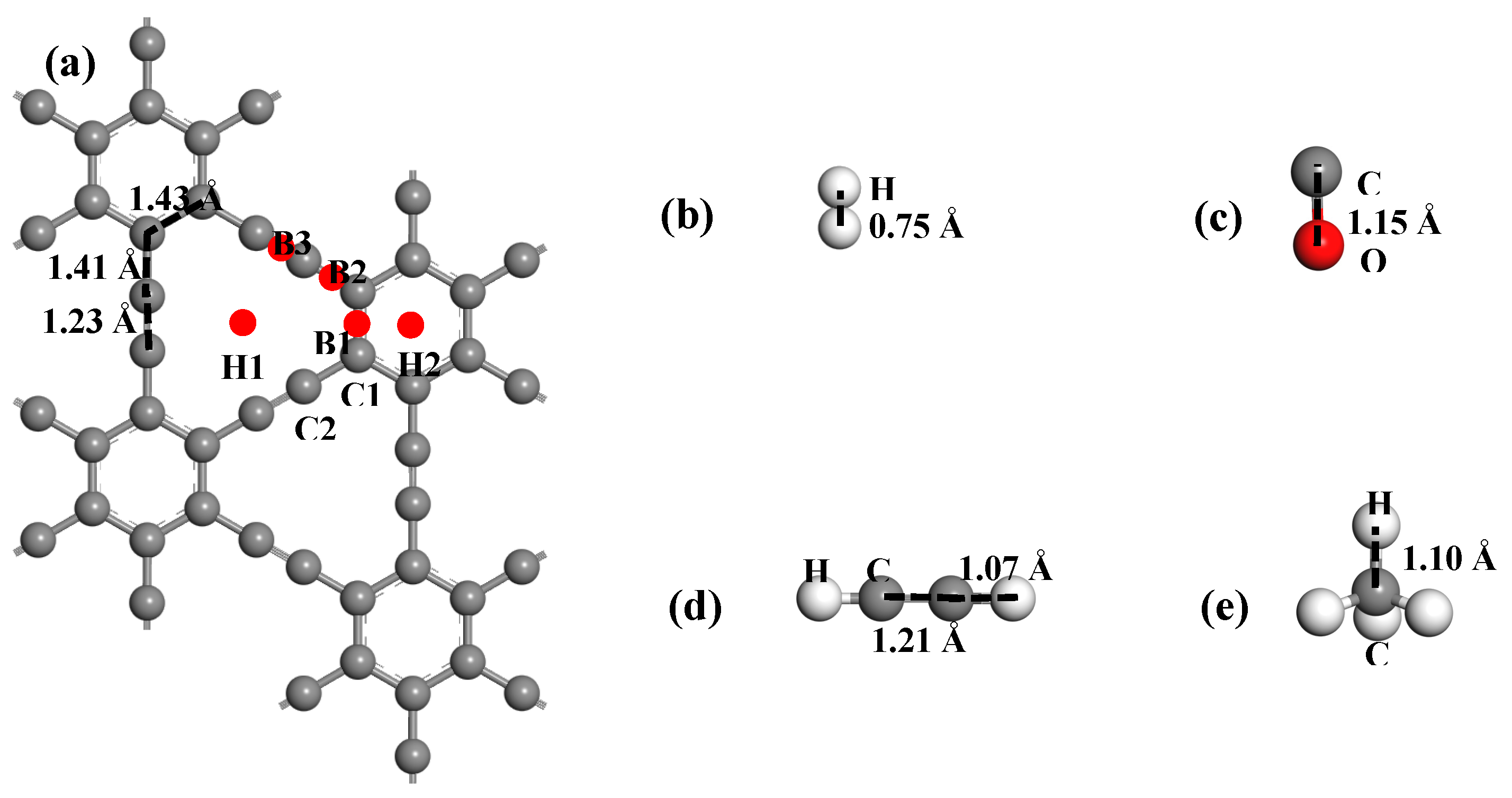
3.1. The Structure of Pd-Graphyne and Dissolved Gas in Transformer Oil
3.2. Adsorption of Gas Molecule on Pd-Graphyne
3.3. Electronic Properties of Pd-Graphyne and Gas Sensing Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Zhu, H. Recent developments in graphene-based membranes: Structure, mass-transport mechanism and potential applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Z.; Han, C.; He, J.; Ni, Z.; Chen, W. Two-dimensional transition metal dichalcogenides: Interface and defect engineering. Chem. Soc. Rev. 2018, 47, 3100–3128. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Rajkamal, A.; Thapa, R. Carbon allotropes as anode material for lithium-ion batteries. Adv. Mater. Technol. 2019. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: Mxenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal–organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wang, N.; Xue, Y.; Zuo, Z.; Liu, H.; Li, Y. Progress in research into 2d graphdiyne-based materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Fukui, N.; Maeda, H.; Matsuoka, R.; Toyoda, R.; Nishihara, H. The accelerating world of graphdiynes. Adv. Mater. 2019, 1804211. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Puigdollers, A.; Gamallo, P. DFT study of the role of N- and B-doping on structural, elastic and electronic properties of α-, β- and γ-graphyne. Carbon 2017, 114, 301–310. [Google Scholar] [CrossRef]
- Kim, B.G.; Choi, H.J. Graphyne: Hexagonal network of carbon with versatile Dirac cones. Phys. Rev. B 2012, 86, 115435. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, Y.; Tan, Y.; Liu, S.; Cheng, Z.; Shen, Z. Graphyne doped with transition-metal single atoms as effective bifunctional electrocatalysts for water splitting. Appl. Surf. Sci. 2019, 492, 8–15. [Google Scholar] [CrossRef]
- He, T.; Matta, S.K.; Du, A. Single tungsten atom supported on N-doped graphyne as a high-performance electrocatalyst for nitrogen fixation under ambient conditions. Phys. Chem. Chem. Phys. 2019, 21, 1546–1551. [Google Scholar] [CrossRef]
- Kim, S.; Ruiz Puigdollers, A.; Gamallo, P.; Viñes, F.; Lee, J.Y. Functionalization of γ-graphyne by transition metal adatoms. Carbon 2017, 120, 63–70. [Google Scholar] [CrossRef]
- He, J.; Zhou, P.; Jiao, N.; Ma, S.Y.; Zhang, K.W.; Wang, R.Z.; Sun, L.Z. Magnetic exchange coupling and anisotropy of 3d transition metal nanowires on graphyne. Sci. Rep. 2014, 4, 4014. [Google Scholar] [CrossRef]
- Kang, B.; Shi, H.; Wang, F.-F.; Lee, J.Y. Importance of doping site of B, N, and O in tuning electronic structure of graphynes. Carbon 2016, 105, 156–162. [Google Scholar] [CrossRef]
- Kang, B.; Ai, H.; Lee, J.Y. Single-atom vacancy induced changes in electronic and magnetic properties of graphyne. Carbon 2017, 116, 113–119. [Google Scholar] [CrossRef]
- Chen, Z.W.; Wen, Z.; Jiang, Q. Rational design of Ag38 cluster supported by graphdiyne for catalytic co oxidation. J. Phys. Chem. C 2017, 121, 3463–3468. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y.; Zhang, G.; Yang, J. Density functional theory study of small Ag cluster adsorbed on graphyne. Appl. Surf. Sci. 2019, 465, 93–102. [Google Scholar] [CrossRef]
- Seif, A.; López, M.J.; Granja-DelRío, A.; Azizi, K.; Alonso, J.A. Adsorption and growth of palladium clusters on graphdiyne. Phys. Chem. Chem. Phys. 2017, 19, 19094–19102. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, X.; Zhang, Y.; Gao, H.; Wang, Y.; Shi, Q.; Rao, D.; Liu, Y.; Deng, K.; Lu, R. Graphdiyne as a high-efficiency membrane for separating oxygen from harmful gases: A first-principles study. ACS Appl. Mater. Interfaces 2016, 8, 28166–28170. [Google Scholar] [CrossRef]
- Lu, Z.; Lv, P.; Ma, D.; Yang, X.; Li, S.; Yang, Z. Detection of gas molecules on single Mn adatom adsorbed graphyne: A DFT-D study. J. Phys. D-Appl. Phys. 2018, 51, 065109. [Google Scholar] [CrossRef]
- Omidvar, A.; Mohajeri, A. Decorated graphyne and its boron nitride analogue as versatile nanomaterials for CO detection. Mol. Phys. 2015, 113, 3900–3908. [Google Scholar] [CrossRef]
- Peyghan, A.A.; Rastegar, S.F.; Hadipour, N.L. DFT study of NH3 adsorption on pristine, Ni- and Si-doped graphynes. Phys. Lett. A 2014, 378, 2184–2190. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Z.; Wu, W.; Liu, Y.; Zhou, Z. Adsorption of NOx (x = 1, 2) gas molecule on pristine and B atom embedded γ-graphyne based on first-principles study. Appl. Surf. Sci. 2018, 455, 484–491. [Google Scholar] [CrossRef]
- Nagarajan, V.; Srimathi, U.; Chandiramouli, R. First-principles insights on detection of dimethyl amine and trimethyl amine vapors using graphdiyne nanosheets. Comput. Theor. Chem. 2018, 1123, 119–127. [Google Scholar] [CrossRef]
- Ma, D.W.; Li, T.; Wang, Q.; Yang, G.; He, C.; Ma, B.; Lu, Z. Graphyne as a promising substrate for the noble-metal single-atom catalysts. Carbon 2015, 95, 756–765. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Cui, H.; Tang, J.; Pi, S.; Cui, Z.; Li, Y.; Zhang, Y. High selectivity n-type InSe monolayer toward decomposition products of sulfur hexafluoride: A density functional theory study. Appl. Surf. Sci. 2019, 479, 852–862. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Xiong, H.; Li, Y.; Tang, J.; Xiao, S.; Zhang, D. A first-principles study of the SF6 decomposed products adsorbed over defective WS2 monolayer as promising gas sensing device. IEEE Trans. Device Mater. Reliab. 2019, 19, 473–483. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Pi, S.; Li, Y.; Cui, Z. High Selective SO2 Gas Sensor Based on Monolayer β-AsSb to Detect SF6 Decompositions. IEEE Sens. J. 2018, 19, 1215–1223. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Zhang, X.; Li, Y.; Liu, H. Detecting decompositions of sulfur hexafluoride using MoS2 monolayer as gas sensor. IEEE Sens. J. 2018, 19, 39–46. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and Cu decorated hexagonal Inn monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H.; Pi, S. Theoretical study of monolayer PtSe2 as outstanding gas sensor to detect SF6 decompositions. IEEE Electron. Device Lett. 2018, 39, 1405–1408. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Li, P.; Cao, Y. Room-temperature SO2 gas-sensing properties based on a metal-doped MoS2 nanoflower: An experimental and density functional theory investigation. J. Mater. Chem. A 2017, 5, 20666–20677. [Google Scholar] [CrossRef]
- Chu, J.; Wang, X.; Wang, D.; Yang, A.; Lv, P.; Wu, Y.; Rong, M.; Gao, L. Highly selective detection of sulfur hexafluoride decomposition components H2S and SOF2 employing sensors based on tin oxide modified reduced graphene oxide. Carbon 2018, 135, 95–103. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Li, Y.; Chen, D.; Zhang, Y. First-principles insight into Ni-doped Inn monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Lu, Z.; Wei, Z.; Xu, L.; Gui, Y. Recent advances of SnO2-based sensors for detecting fault characteristic gases extracted from power transformer oil. Front. Chem. 2018, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, F.; Sun, Q.; Ye, H.; Jiang, Q. Application of polycrystalline SnO2 sensor chromatographic system to detect dissolved gases in transformer oil. Sens. Actuator B-Chem. 2018, 267, 636–646. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Yaqoob, U.; Chung, G.-S. Dissolved hydrogen gas analysis in transformer oil using Pd catalyst decorated on ZnO nanorod array. Sens. Actuator B-Chem. 2016, 226, 90–95. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Gao, T.; Su, X.; Wan, F. Pd-doped SnO2-based sensor detecting characteristic fault hydrocarbon gases in transformer oil. J. Nanomater. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Xiong, H.; Chen, D.; Cheng, H.; Tang, J.; Tian, Y.; Xiao, S. Dissolved Gas Analysis in Transformer Oil Using Pt-Doped WSe2 Monolayer Based on First Principles Method. IEEE Access 2019, 7, 72012–72019. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Tan, S.; Liu, T.; Cui, H. Adsorption characteristic of Rh-doped MoSe2 monolayer towards H2 and C2H2 for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 487, 930–937. [Google Scholar] [CrossRef]
- Delley, B. Dmol3 DFT studies: From molecules and molecular environments to surfaces and solids. Comput. Mater. Sci. 2000, 17, 122–126. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA- type density functional constructed with a long- range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chem. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.Y. Graphynes as promising cathode material of fuel cell: Improvement of oxygen reduction efficiency. J. Phys. Chem. C 2014, 118, 12035–12040. [Google Scholar] [CrossRef]
- Li, S.S. Semiconductor Physical Electronics; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef]
- Patel, K.; Roondhe, B.; Dabhi, S.D.; Jha, P.K. A new flatland buddy as toxic gas scavenger: A first principles study. J. Hazard. Mater. 2018, 351, 337–345. [Google Scholar] [CrossRef]







| Adsorption Site | Ebind (eV) | QT (e) |
|---|---|---|
| H1 site | −2.45 | +0.363 |
| H2 site | −1.08 | +0.323 |
| B1 site | −1.59 | +0.344 |
| B2 site | −1.52 | +0.315 |
| B3 site | −1.76 | +0.273 |
| Structure | Eads (eV) | QT (e) |
|---|---|---|
| Pd-graphyne/H2 | −0.08 | −0.059 |
| Pd-graphyne/CO | −1.11 | −0.080 |
| Pd-graphyne/C2H2 | −0.16 | −0.015 |
| Pd-graphyne/CH4 | −0.13 | −0.063 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fang, R.; Chen, D.; Zhang, G. Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation. Nanomaterials 2019, 9, 1490. https://doi.org/10.3390/nano9101490
Zhang X, Fang R, Chen D, Zhang G. Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation. Nanomaterials. 2019; 9(10):1490. https://doi.org/10.3390/nano9101490
Chicago/Turabian StyleZhang, Xiaoxing, Rongxing Fang, Dachang Chen, and Guozhi Zhang. 2019. "Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation" Nanomaterials 9, no. 10: 1490. https://doi.org/10.3390/nano9101490
APA StyleZhang, X., Fang, R., Chen, D., & Zhang, G. (2019). Using Pd-Doped γ-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation. Nanomaterials, 9(10), 1490. https://doi.org/10.3390/nano9101490