Graphene IoNanofluids, Thermal and Structural Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. TGA
3.2. Phase Change Characterization
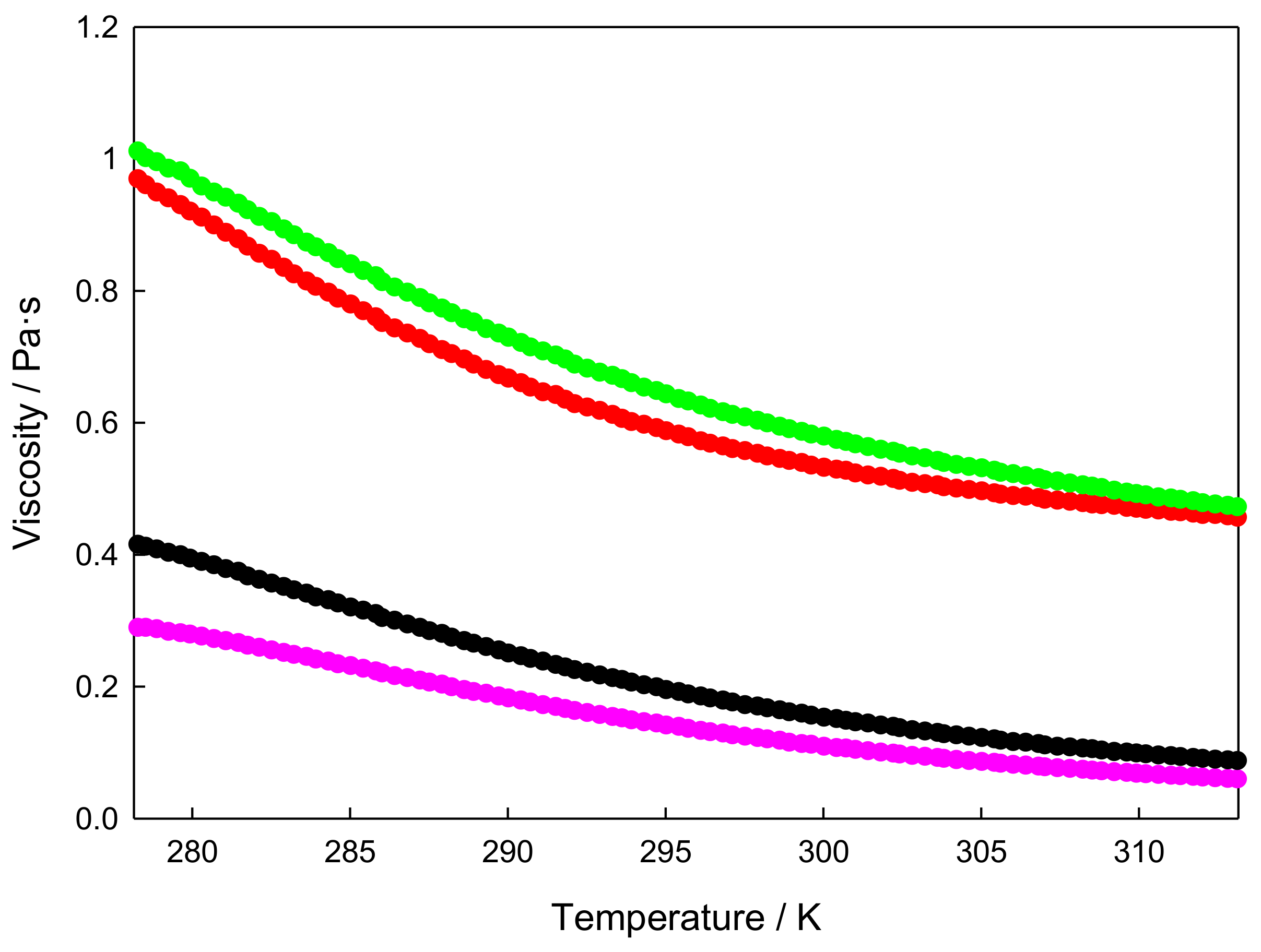
3.3. Isobaric Heat Capacity
3.4. Degree of Subcooling
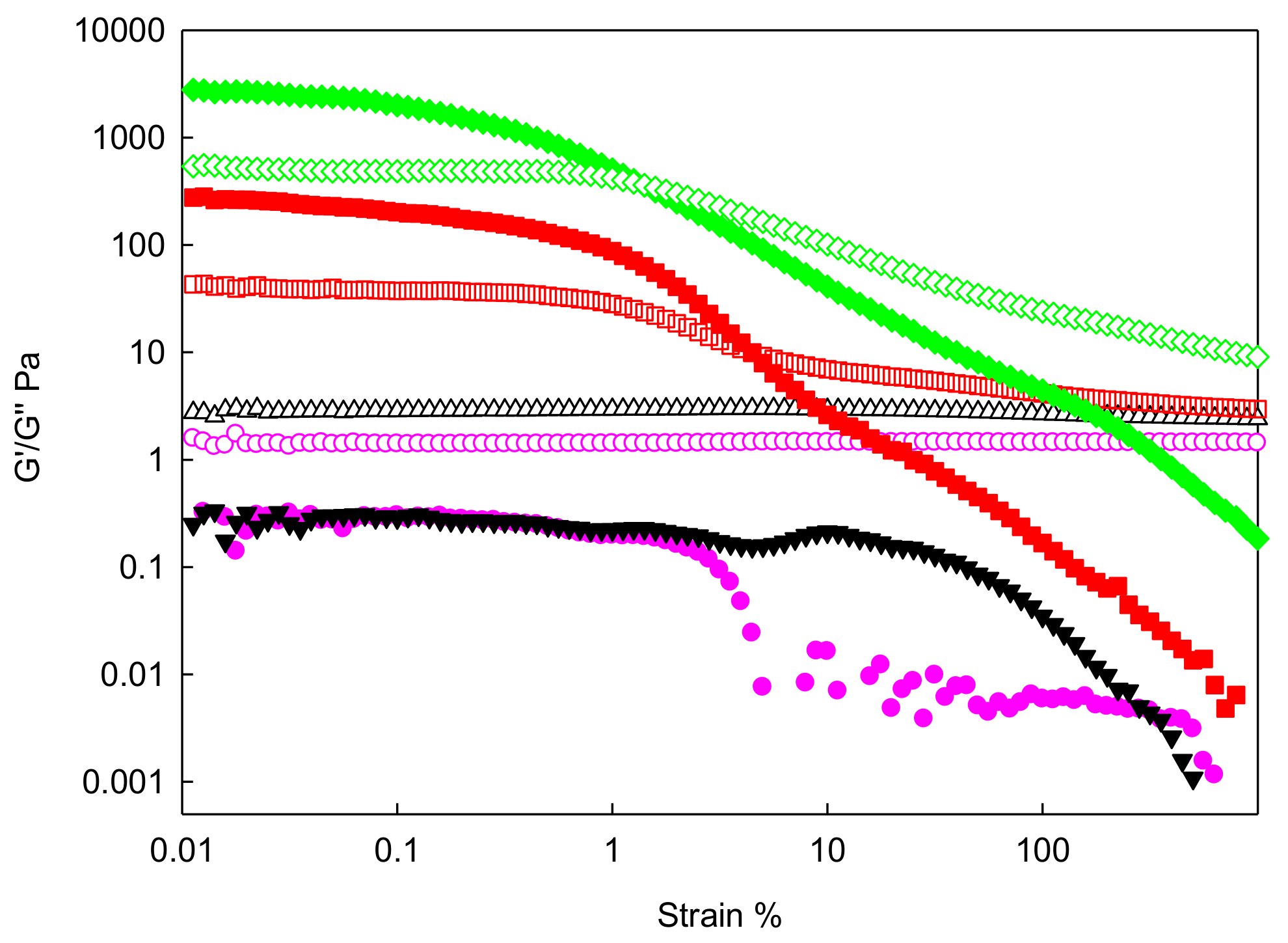
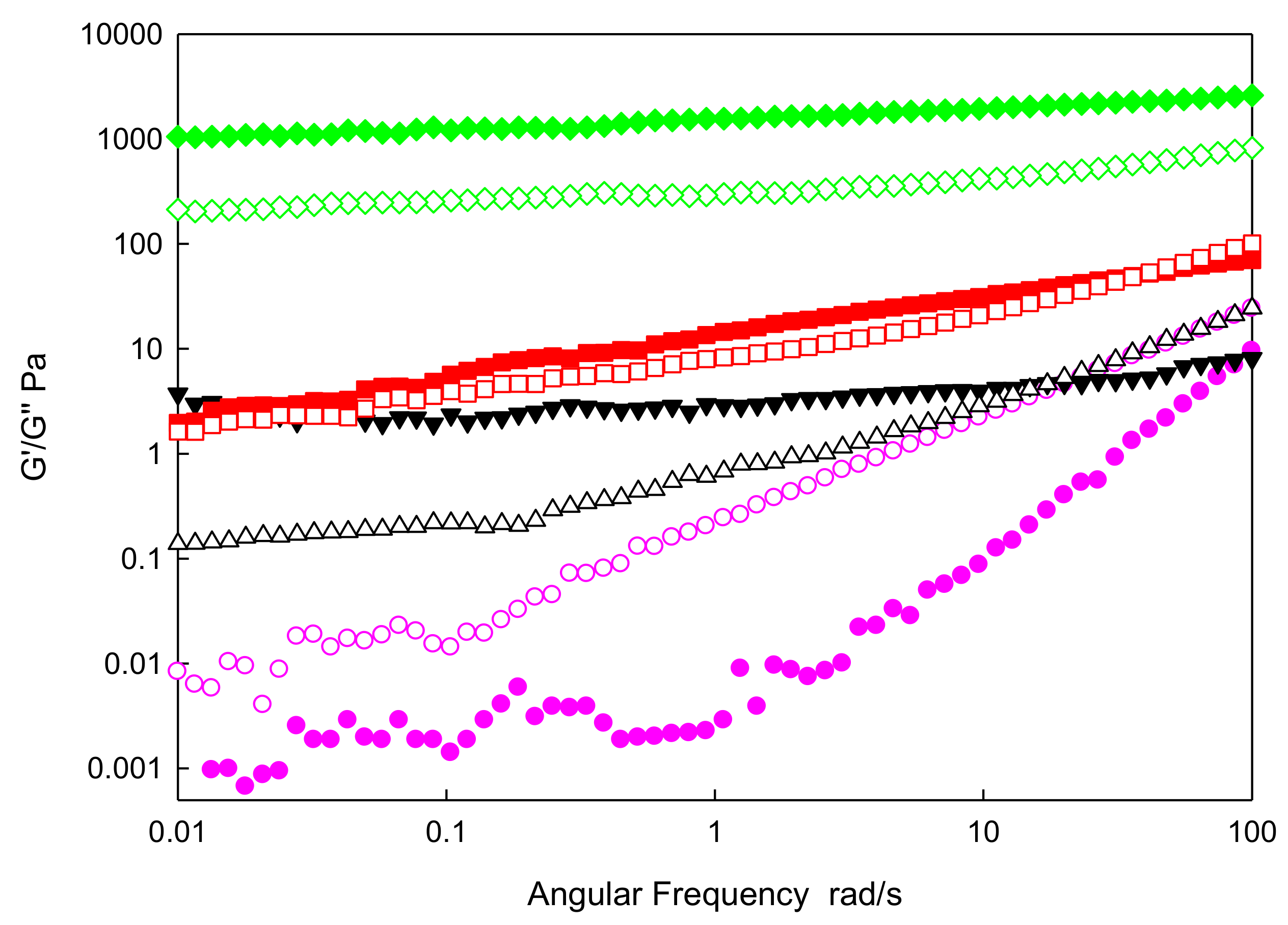
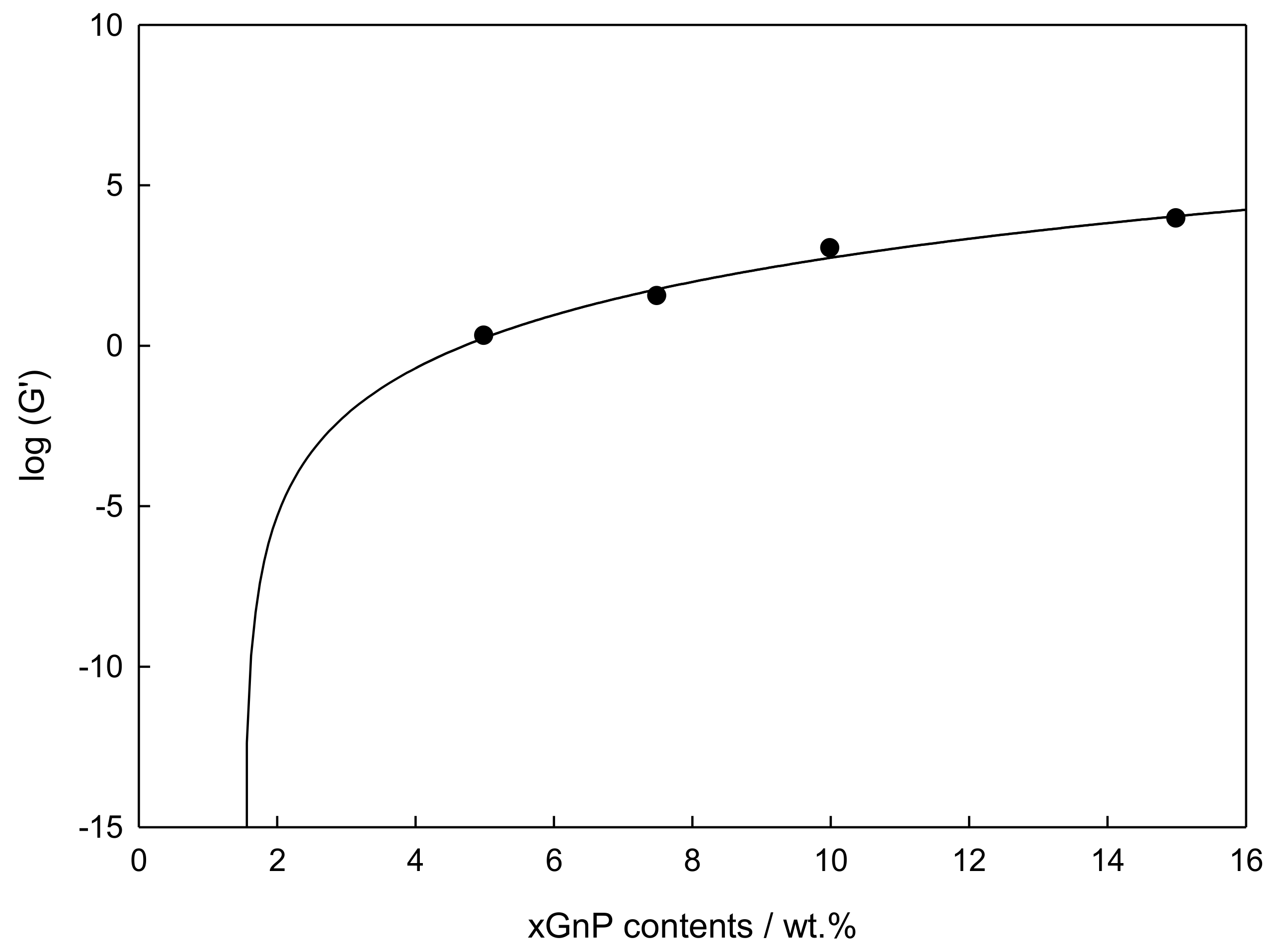
3.5. Viscoelastic Measurements
Oscillatory Rheology
- (1)
- Strain Sweep
- (2)
- Frequency Sweep
- (3)
- Graphene Percolation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Araújo, J.M.; Florindo, C.; Pereiro, A.B.; Vieira, N.S.; Matias, A.A.; Duarte, C.M.; Rebelo, L.P.; Marrucho, I.M. Cholinium-based ionic liquids with pharmaceutically active anions. RSC Adv. 2014, 4, 28126–28132. [Google Scholar] [CrossRef]
- Shirakawa, S.; Tanaka, Y.; Maruoka, K. Development of a recyclable fluorous chiral phase-transfer catalyst: Application to the catalytic asymmetric synthesis of α-amino acids. Org. Lett. 2004, 6, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, Y.; Iwamoto, K.; Furutani, H.; Matsushita, Y.; Abe, Y.; Matsumoto, K.; Monda, K.; Hayase, S.; Kawatsura, M.; Itoh, T. Preparation of novel hydrophobic fluorine-substituted-alkyl sulfate ionic liquids and application as an efficient reaction medium for lipase-catalyzed reaction. Tetrahedron Lett. 2006, 47, 1801–1804. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Pastoriza-Gallego, M.J.; Shimizu, K.; Marrucho, I.M.; Lopes, J.N.C.; Piñeiro, M.M.; Rebelo, L.P.N. On the formation of a third, nanostructured domain in ionic liquids. J. Phys. Chem. B 2013, 117, 10826–10833. [Google Scholar] [CrossRef] [PubMed]
- Hameed, N.; Dumée, L.F.; Allioux, F.M.; Reghat, M.; Church, J.S.; Naebe, M.; Magniez, K.; Parameswaranpillai, J.; Fox, B.L. Graphene based room temperature flexible nanocomposites from permanently cross-linked networks. Sci. Rep. 2018, 8, 2803. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Merino, C.; Perez-Rodriguez, M.; Pereiro, A.B.; Piñeiro, M.M.; Pastoriza-Gallego, M.J. Tailoring nanofluid thermophysical profile through graphene nanoplatelets surface functionalization. ACS Omega 2018, 3, 744–752. [Google Scholar] [CrossRef]
- Álvarez-Regueiro, E.; Vallejo, J.P.; Fernández-Seara, J.; Fernández, J.; Lugo, L. Experimental convection heat transfer analysis of a nano-enhanced industrial coolant. Nanomaterials 2019, 9, 267. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Tian, Z.; Goodarzi, M.; Bandarra Filho, E.P.; Arjomandi, M. Thermal assessment of nano-particulate graphene-water/ethylene glycol (WEG 60:40) nano-suspension in a compact heat exchanger. Energies 2019, 12, 1929. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R. Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphene nanoplatelets-methanol nano-suspension. Renew. Energy 2019, 142, 364–372. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Yang, B.; Pourmehran, O.; Arjomandi, M.; Ghomashchi, R. Fluid and heat transfer characteristics of aqueous graphene nanoplatelet (GNP) nanofluid in a microchannel. Int. Commun. Heat Mass Transf. 2019, 107, 24–33. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Araújo, J.M.; Teixeira, F.S.; Marrucho, I.M.; Piñeiro, M.M.; Rebelo, L.P.N. Aggregation behavior and total miscibility of fluorinated ionic liquids in water. Langmuir 2015, 31, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Vieira, N.S.; Bastos, J.C.; Hermida-Merino, C.; Pastoriza-Gallego, M.J.; Rebelo, L.P.; Piñeiro, M.M.; Araújo, J.M.; Pereiro, A.B. Aggregation and phase equilibria of fluorinated ionic liquids. J. Mol. Liq. 2019, 285, 386–396. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Araújo, J.M.; Pereiro, A.B.; Vega, L.F. Insights into the influence of the molecular structures of fluorinated ionic liquids on their thermophysical properties. A soft-SAFT based approach. Phys. Chem. Chem. Phys. 2019, 21, 6362–6380. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Tomé, L.C.; Martinho, S.; Rebelo, L.P.N.; Marrucho, I.M. Gas permeation properties of fluorinated ionic liquids. Ind. Eng. Chem. Res. 2013, 52, 4994–5001. [Google Scholar] [CrossRef]
- Alves, M.; Vieira, N.S.; Rebelo, L.P.N.; Araújo, J.M.; Pereiro, A.B.; Archer, M. Fluorinated ionic liquids for protein drug delivery systems: Investigating their impact on the structure and function of lysozyme. Int. J. Pharm. 2017, 526, 309–320. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Pastoriza-Gallego, M.J.; Araújo, J.M.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Piñeiro, M.M.; Shimizu, K.; Pereiro, A.B. Influence of nanosegregation on the phase behavior of fluorinated ionic liquids. J. Phys. Chem. C 2017, 121, 5415–5427. [Google Scholar] [CrossRef]
- Żyła, G.; Vallejo, J.P.; Fal, J.; Lugo, L. Nanodiamonds—Ethylene glycol nanofluids: Experimental investigation of fundamental physical properties. Int. J. Heat Mass Transf. 2018, 121, 1201–1213. [Google Scholar] [CrossRef]
- Żyła, G.; Vallejo, J.P.; Lugo, L. Isobaric heat capacity and density of ethylene glycol based nanofluids containing various nitride nanoparticle types: An experimental study. J. Mol. Liq. 2018, 261, 530–539. [Google Scholar] [CrossRef]
- Matsuoka, M.; Ozawa, R. Determination of solid-liquid phase equilibria of binary organic systems by differential scanning calorimetry. J. Cryst. Growth 1989, 96, 596–604. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Lugo, L. (Solid + liquid) phase equilibria and heat capacity of (diphenyl ether + biphenyl) mixtures used as thermal energy storage materials. J. Chem. Thermodyn. 2014, 74, 43–50. [Google Scholar] [CrossRef]
- Hermida-Merino, C.; Pérez-Rodríguez, M.; Pineiro, M.M.; Pastoriza-Gallego, M.J. Evidence of viscoplastic behavior of exfoliated graphite nanofluids. Soft Matter 2016, 12, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Influence of six carbon-based nanomaterials on the rheological properties of nanofluids. Nanomaterials 2019, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.P.; Żyła, G.; Fernández-Seara, J.; Lugo, L. Rheological behaviour of functionalized graphene nanoplatelet nanofluids based on water and propylene glycol: Water mixtures. Int. Commun. Heat Mass Transf. 2018, 99, 43–53. [Google Scholar] [CrossRef]
- Riazi, H.; Murphy, T.; Webber, G.B.; Atkin, R.; Tehrani, S.S.M.; Taylor, R.A. Specific heat control of nanofluids: A critical review. Int. J. Therm. Sci. 2016, 107, 25–38. [Google Scholar] [CrossRef]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Jayavel, R.; Kalaiselvam, S. Study on thermal properties of organic ester phase-change material embedded with silver nanoparticles. J. Therm. Anal. Calorim. 2013, 114, 845–858. [Google Scholar] [CrossRef]
- Gracia-Fernandez, C.; Gómez-Barreiro, S.; López-Beceiro, J.; Naya, S.; Artiaga, R. Characterization of MWCNT/TPU systems by large amplitude oscillation shear. J. Therm. Anal. Calorim. 2014, 115, 1727–1731. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Auad, M.L.; Bellesi, N.E.; Nutt, S.R.; Aranguren, M.I. Cellulose micro/nanocrystals reinforced polyurethane. J. Mater. Res. 2006, 21, 870–881. [Google Scholar] [CrossRef]





| [C2C1py][C4F9SO3] | XGNP/ [C2C1py][C4F9SO3] 1 wt% | XGNP/ [C2C1py][C4F9SO3] 5 wt% | XGNP/ [C2C1py][C4F9SO3] 10 wt% | |
|---|---|---|---|---|
| TONSET | 685 K | 684 K | 688 K | 686 K |
| MAXIMUM PEAK T | 710 K | 713 K | 705 K | 712 K |
| [C2C1py][C4F9SO3] | 1 wt% | 5 wt% | 10 wt% | |
|---|---|---|---|---|
| T (K) | cp (J·g−1·K−1) | cp (J·g−1·K−1) | cp (J·g−1·K−1) | cp (J·g−1·K−1) |
| 293 | 1.296 | 1.321 | 1.359 | 1.401 |
| 313 | 1.334 | 1.360 | 1.403 | 1.446 |
| 333 | 1.367 | 1.391 | 1.442 | 1.482 |
| 353 | 1.397 | 1.421 | 1.472 | 1.511 |
| Concentration, wt% | TCrys (K) | Tm (K) | ΔHm (J · g−1) | ΔHCrys(J·g−1) |
|---|---|---|---|---|
| [C2C1py][C4F9SO3] | 250 | 278 | 38.14 | 34.76 |
| 1 wt% xGnP/[C2C1py][C4F9SO3] | 268 | 280 | 41.33 | 40.45 |
| 5 wt% xGnP/[C2C1py][C4F9SO3] | 258 | 277 | 41.16 | 39.47 |
| 10 wt% xGnP/[C2C1py][C4F9SO3] | 255 | 274 | 40.18 | 39.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermida-Merino, C.; Pereiro, A.B.; Araújo, J.M.M.; Gracia-Fernández, C.; Vallejo, J.P.; Lugo, L.; Piñeiro, M.M. Graphene IoNanofluids, Thermal and Structural Characterization. Nanomaterials 2019, 9, 1549. https://doi.org/10.3390/nano9111549
Hermida-Merino C, Pereiro AB, Araújo JMM, Gracia-Fernández C, Vallejo JP, Lugo L, Piñeiro MM. Graphene IoNanofluids, Thermal and Structural Characterization. Nanomaterials. 2019; 9(11):1549. https://doi.org/10.3390/nano9111549
Chicago/Turabian StyleHermida-Merino, C., A.B. Pereiro, J.M.M. Araújo, C. Gracia-Fernández, Javier P. Vallejo, Luis Lugo, and M.M. Piñeiro. 2019. "Graphene IoNanofluids, Thermal and Structural Characterization" Nanomaterials 9, no. 11: 1549. https://doi.org/10.3390/nano9111549







