Inhibitory Effects of Cu2O/SiO2 on the Growth of Microcystis aeruginosa and Its Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Cu2O/SiO2
2.2. Algae Growth Inhibition Experiment
2.2.1. Algae Cultures
2.2.2. Cu2O/SiO2 Inhibit Algae Growth Experiment
2.3. Mechanism Experiment of Cu2O/SiO2 Inhibiting Algae Growth
2.3.1. The Effect of Cu2+
2.3.2. The Effect on Chlorophyll a
2.3.3. The Release of ·OH
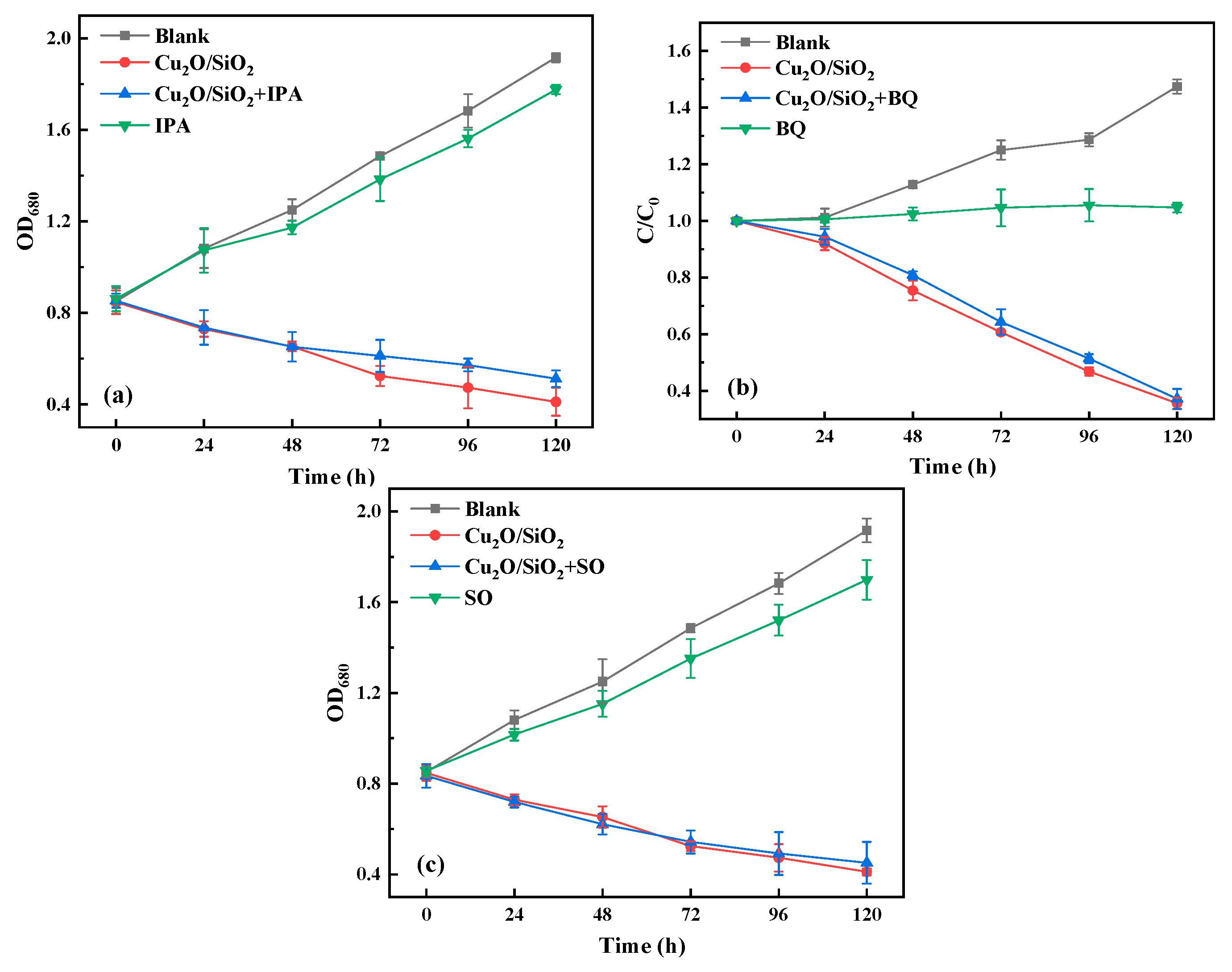
2.3.4. Active Species Trapping Experiments
2.3.5. The Effect on Antioxidant Enzyme
2.3.6. Extracellular Organic Matter (EOM) & Intracellular Organic Matter (IOM)
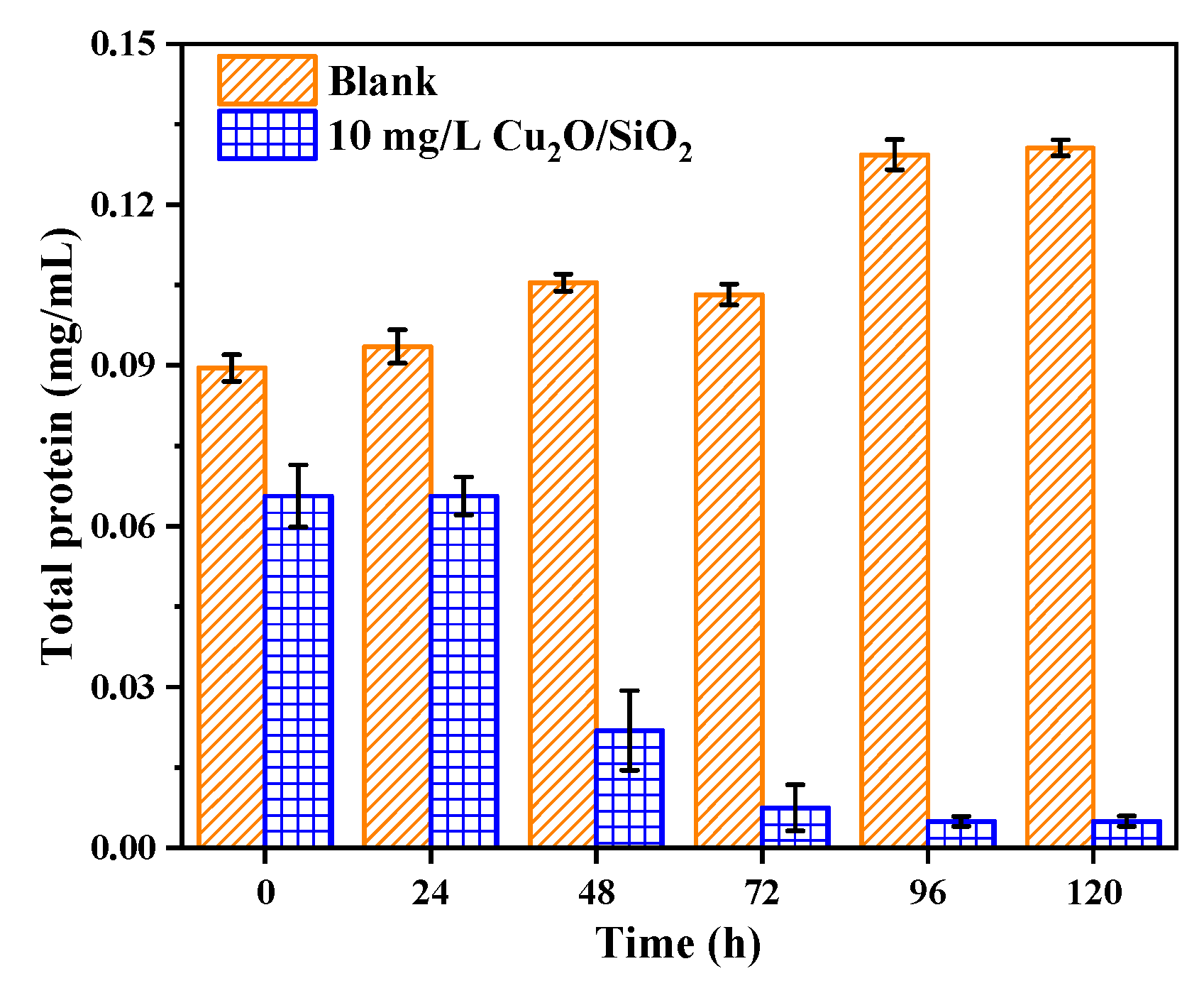
2.3.7. The Effect on Total Protein
2.3.8. The Effect on Phycobiliprotein
3. Results and Discussion
3.1. Characterization of Cu2O/SiO2
3.1.1. X-ray Diffraction (XRD) & Scanning Electron Microscope (SEM)
3.1.2. Ultraviolet-Visible Spectroscopy (UV-Vis)
3.2. Growth Inhibition of Cu2O/SiO2 on Algal Cells
3.3. Mechanism of Cu2O/SiO2 Inhibiting Algae Growth
3.3.1. Metal Ions
3.3.2. Chlorophyll a
3.3.3. ·OH Assays
3.3.4. Active Species Trapping Experiments
3.3.5. Antioxidant Enzyme
3.3.6. EOM & IOM
3.3.7. Total Protein
3.3.8. Phycobiliprotein
3.3.9. Mechanism of Cu2O/SiO2 Inhibit the Growth of Algal Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.Y.; Yu, Y.H.; Feng, W.S.; Pan, G.; Chen, H.; Chen, J.; Yang, B.; Li, X.M.; Zhang, X. Plankton Community Succession in Artificial Systems Subjected to Cyanobacterial Blooms Removal using Chitosan-Modified Soils. Microb. Ecol. 2009, 58, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, B.; Islam, M.A.; Beardall, J.; Zhang, X.W.; McCarthy, D. Effective electrochemical inactivation of Microcystis aeruginosa and degradation of microcystins via a novel solid polymer electrolyte sandwich. Chem. Eng. J. 2018, 350, 616–626. [Google Scholar] [CrossRef]
- Starling, F.L.D.R. Control of eutrophication by silver carp (Hypophthalmichthys molitrix) in the tropical Paranoá Reservoir (Brasília, Brazil): A mesocosm experiment. Hydrobiologia 1993, 257, 143–152. [Google Scholar] [CrossRef]
- Kondzior, P.; Butarewicz, A. Effect of Heavy Metals (Cu and Zn) on the Content of Photosynthetic Pigments in the Cells of Algae Chlorella vulgaris. J. Ecol. Eng. 2018, 19, 18–28. [Google Scholar] [CrossRef]
- Park, J.; Church, J.; Son, Y.; Kim, K.T.; Lee, W.H. Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason. Sonochem. 2017, 38, 326–334. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhang, X.Z.; Chen, Y.S.; Sommerfeld, M.; Hu, Q. Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii. Chemosphere 2008, 73, 1121–1128. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.; Wang, Z.; Dai, Y.; Xing, B. Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae. Water Res. 2017, 111, 18–27. [Google Scholar] [CrossRef]
- Gu, N.; Gao, J.; Wang, K.; Li, B.; Dong, W.; Ma, Y. Microcystis aeruginosa inhibition by Zn-Fe-LDHs as photocatalyst under visible light. J. Taiwan Inst. Chem. Eng. 2016, 64, 189–195. [Google Scholar] [CrossRef]
- Guo, R.; Wang, H.; Suh, Y.S.; Ki, J.S. Transcriptomic profiles reveal the genome-wide responses of the harmful dinoflagellate Cochlodinium polykrikoides when exposed to the algicide copper sulfate. BMC Genom. 2016, 17, 29. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B-Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Kiwi, J. Polystyrene CuO/Cu2O uniform films inducing MB-degradation under sunlight. Catal. Today 2017, 284, 77–83. [Google Scholar] [CrossRef]
- Guo, S.; Fang, Y.; Dong, S.; Wang, E. Templateless, surfactantless, electrochemical route to a cuprous oxide microcrystal: From octahedra to monodisperse colloid spheres. Inorg. Chem. 2007, 46, 9537–9539. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Neti, N.R.; Sinha, A.S.K.; Srivastava, O.N. Growth of different nanostructures of Cu2O (nanothreads, nanowires, and nanocubes) by simple electrolysis based oxidation of copper. J. Phys. Chem. C 2007, 111, 1638–1645. [Google Scholar] [CrossRef]
- Cao, C.H.; Xiao, L.; Chen, C.H.; Cao, Q.H. Synthesis of novel Cu2O/BiOCl heterojunction nanocomposites and their enhanced photocatalytic activity under visible light. Appl. Surf. Sci. 2015, 357, 1171–1179. [Google Scholar] [CrossRef]
- Garcia, R.; Tello, M.; Moulin, J.F.; Biscarini, F. Size and shape controlled growth of molecular nanostructures on silicon oxide templates. Nano Lett. 2004, 4, 1115–1119. [Google Scholar] [CrossRef]
- Duong, T.T.; Le, T.S.; Tran, T.T.H.; Nguyen, T.K.; Ho, C.T.; Dao, T.H.; Le, T.P.Q.; Nguyen, H.C.; Dang, D.K.; Le, T.T.H.; et al. Inhibition effect of engineered silver nanoparticles to bloom forming cyanobacteria. Adv. Nat. Sci. Nanosci. 2016, 7, 035018. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Prathna, T.C.; Trivedi, S.; Myneni, R.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat. Toxicol. 2013, 132–133, 34–45. [Google Scholar] [CrossRef]
- Fan, G.D.; Bao, M.C.; Zheng, X.M.; Hong, L.; Zhan, J.J.; Chen, Z.; Qu, F.S. Growth inhibition of harmful cyanobacteria by nanocrystalline Cu-MOF-74: Efficiency and its mechanisms. J. Hazard Mater. 2019, 367, 529–538. [Google Scholar] [CrossRef]
- Loprasert, S.; Vattanaviboon, P.; Praituan, W.; Chamnongpol, S.; Mongkolsuk, S. Regulation of the oxidative stress protective enzymes, catalase and superoxide dismutase in Xanthomonas—A review. Gene 1996, 179, 33–37. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Wiczkowski, A. Oxidative damage and carcinogenesis. Contemp. Oncol. Pozn 2012, 16, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Hamzeh, M.; Dodard, S.; Zhao, Y.H.; Sunahara, G.I. Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ. Toxicol. Pharmacol. 2015, 39, 1074–1080. [Google Scholar]
- Liu, W.; Au, D.W.T.; Anderson, D.M.; Lam, P.K.S.; Wu, R.S.S. Effects of nutrients, salinity, pH and light: Dark cycle on the production of reactive oxygen species in the alga Chattonella marina. J. Exp. Mar. Biol. Ecol. 2007, 346, 76–86. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.J.; Zhou, Y.; Yao, H.Z.; Ahmad, F. Evaluation of the toxicity of ZnO nanoparticles to Chlorella vulgaris by use of the chiral perturbation approach. Anal. Bioanal. Chem. 2014, 406, 3689–3695. [Google Scholar] [CrossRef]
- Ali, E.M.; Khairy, H.M. Environmental assessment of drainage water impacts on water quality and eutrophication level of Lake Idku, Egypt. Environ. Pollut. 2016, 216, 437–449. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; Sonntag, C.V. OH radical formation by ultrasound in aqueous solutions Part I: The chemistry underlying the terephthalate dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Rad, M.; Dehghanpour, S. ZnO as an efficient nucleating agent and morphology template for rapid, facile and scalable synthesis of MOF-46 and ZnO@MOF-46 with selective sensing properties and enhanced photocatalytic ability. RSC Adv. 2016, 6, 61784–61793. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.J.; Zhao, J.F.; Song, J.K.; Wang, J.Y.; Ma, R.R.; Ma, J.X. Solar light-driven photocatalytic destruction of cyanobacteria by F-Ce-TiO2/expanded perlite floating composites. Chem. Eng. J. 2017, 320, 253–263. [Google Scholar] [CrossRef]
- Oukarroum, A.; Zaidi, W.; Samadani, M.; Dewez, D. Toxicity of Nickel Oxide Nanoparticles on a Freshwater Green Algal Strain of Chlorella vulgaris. BioMed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Superior Antibacterial Activity of Fe3O4-TiO2 Nanosheets under Solar Light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Murwanashyaka, T.; Ndikubwimana, T.; Yue, Q.; Zeng, X.H.; Lu, Y.H.; Jing, K.J. Ammonium chloride: A novel effective and inexpensive salt solution for phycocyanin extraction from Arthrospira (Spirulina) platensis. J. Appl. Phycol. 2017, 29, 1261–1270. [Google Scholar] [CrossRef]
- Na, G.; Gao, J.; Li, H.; Wu, Y.; Ma, Y.; Wang, K. Montmorillonite-supported with Cu2O nanoparticles for damage and removal of Microcystis aeruginosa under visible light. Appl. Clay Sci. 2016, 132–133, 79–89. [Google Scholar]
- Zhicong, W.; Dunhai, L.; Hongjie, Q.; Yinxia, L. An integrated method for removal of harmful cyanobacterial blooms in eutrophic lakes. Environ. Pollut. 2012, 160, 34–41. [Google Scholar]
- Fan, G.D.; You, Y.F.; Wang, B.; Wu, S.M.; Zhang, Z.; Zheng, X.M.; Bao, M.C.; Zhan, J.J. Inactivation of harmful cyanobacteria by Ag/AgCl@ZIF-8 coating under visible light: Efficiency and its mechanisms. Appl. Catal. B Environ. 2019, 256, 117866. [Google Scholar] [CrossRef]
- Tian, C.; Guo, T.T.; Liu, R.P.; Jefferson, W.; Liu, H.J.; Jiu-Hui, Q.U. Formation of disinfection by-products by Microcystis aeruginosa intracellular organic matter: Comparison between chlorination and bromination. Huan Jing Ke Xue 2013, 34, 4282–4289. [Google Scholar]
- Qu, F.; Liang, H.; Wang, Z.; Wang, H.; Yu, H.; Li, G. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: Influences of interfacial characteristics of foulants and fouling mechanisms. Water Res. 2012, 46, 1490–1500. [Google Scholar] [CrossRef]
- Tao, Y.; Mao, X.; Hu, J.; Mok, H.O.; Wang, L.; Au, D.W.; Zhu, J.; Zhang, X. Mechanisms of photosynthetic inactivation on growth suppression of Microcystis aeruginosa under UV-C stress. Chemosphere 2013, 93, 637–644. [Google Scholar] [CrossRef]
- Padgett, M.P.; Krogmann, D.W. Large scale preparation of pure phycobiliproteins. Photosynth. Res. 1987, 11, 225–235. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, R.; Chai, Y.; Zhuo, Y.; Hong, C.; Liu, Z.; Su, H. Porous redox-active Cu2O-SiO2 nanostructured film: Preparation, characterization and application for a label-free amperometric ferritin immunosensor. Talanta 2009, 78, 596–601. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Liu, S.; Zhang, Z.; Zhang, H.; Cai, X.; Pan, J.; Liu, J. Effect of TiO2 nanoparticle aggregation on marine microalgae Isochrysis galbana. J. Environ. Sci. China 2018, 66, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hazani, A.A.; Ibrahim, M.M.; Arif, I.A.; Shehata, A.I.; El-Gaaly, G.; Daoud, M.; Fouad, D.; Rizwana, H.; Moubayed, N. Ecotoxicity of Ag-Nanoparticles to Microalgae. J. Pure Appl. Microbiol. 2013, 7, 233–241. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, G.; Bao, M.; Wang, B.; Wu, S.; Luo, L.; Li, B.; Lin, J. Inhibitory Effects of Cu2O/SiO2 on the Growth of Microcystis aeruginosa and Its Mechanism. Nanomaterials 2019, 9, 1669. https://doi.org/10.3390/nano9121669
Fan G, Bao M, Wang B, Wu S, Luo L, Li B, Lin J. Inhibitory Effects of Cu2O/SiO2 on the Growth of Microcystis aeruginosa and Its Mechanism. Nanomaterials. 2019; 9(12):1669. https://doi.org/10.3390/nano9121669
Chicago/Turabian StyleFan, Gongduan, Minchen Bao, Bo Wang, Shimin Wu, Lingxi Luo, Binhui Li, and Jiuhong Lin. 2019. "Inhibitory Effects of Cu2O/SiO2 on the Growth of Microcystis aeruginosa and Its Mechanism" Nanomaterials 9, no. 12: 1669. https://doi.org/10.3390/nano9121669





