Synthesis and Luminescence Properties of Core-Shell-Shell Composites: SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
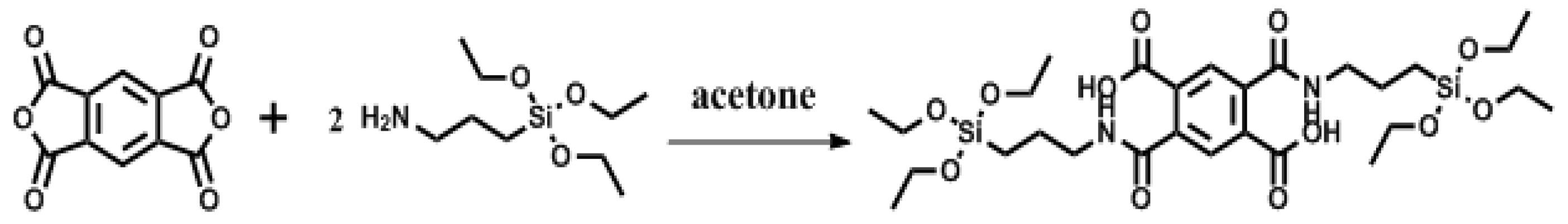
2.2. Synthesis of Organic Ligand (2,5-bis((3-(Triethoxysilyl)propyl)carbamoyl)terephthalic Acid (PMDA-Si))
2.3. Synthesis of Silica Cores
2.4. Synthesis of SiO2@PMDA-Si
2.5. Synthesis of SiO2@PMDA-Si-Tb and SiO2@PMDA-Si-Tb-phen
2.6. Synthesis of SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2
2.7. Characterization and Apparatus
2.8. Lifetime Analysis
3. Results and Discussion
3.1. The Formation Mechanism and Design Idea of Core-Shell and Core-Shell-Shell Composites
3.2. Morphology and Structure
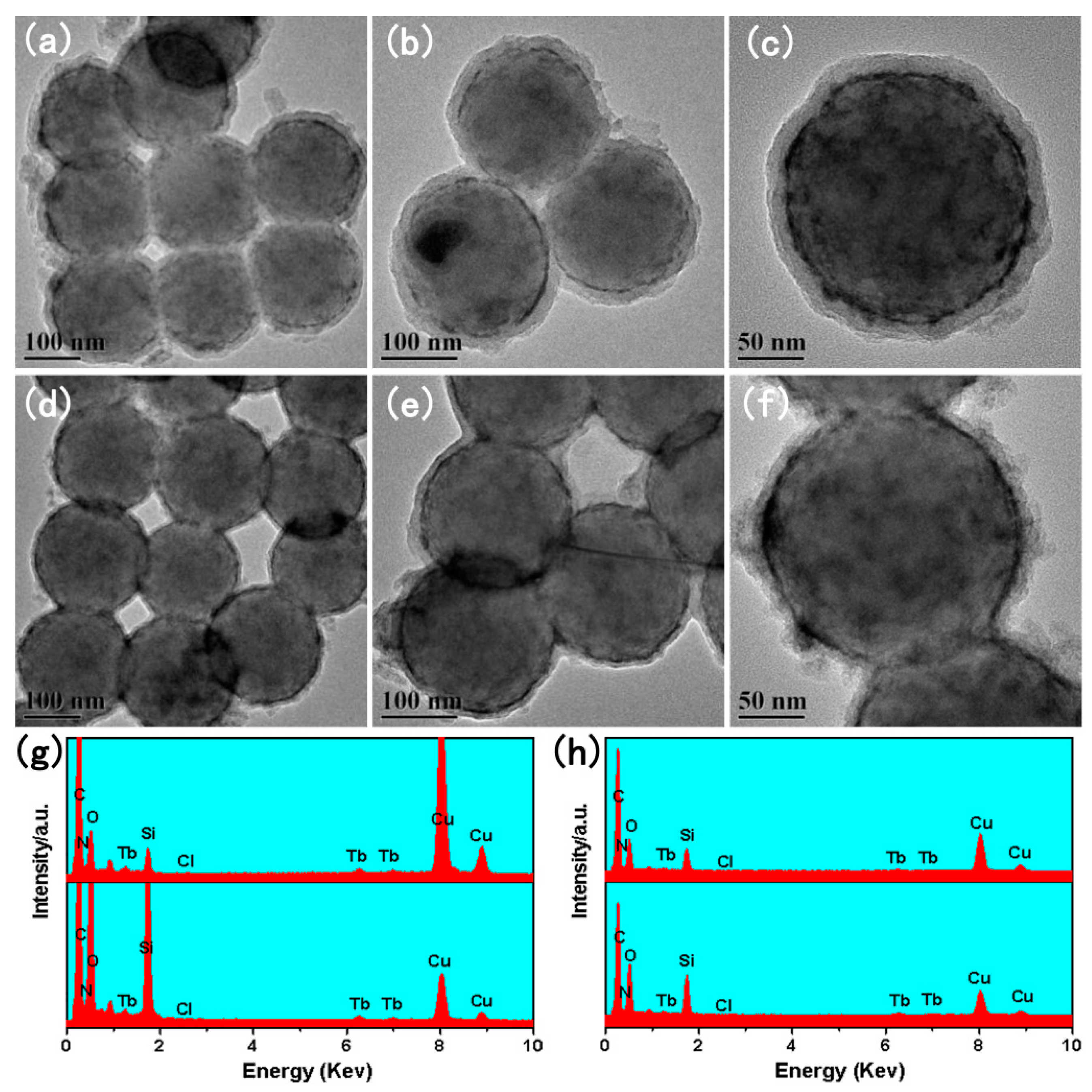
3.2.1. The TEM of SiO2@PMDA-Si-Tb and SiO2@PMDA-Si-Tb-phen
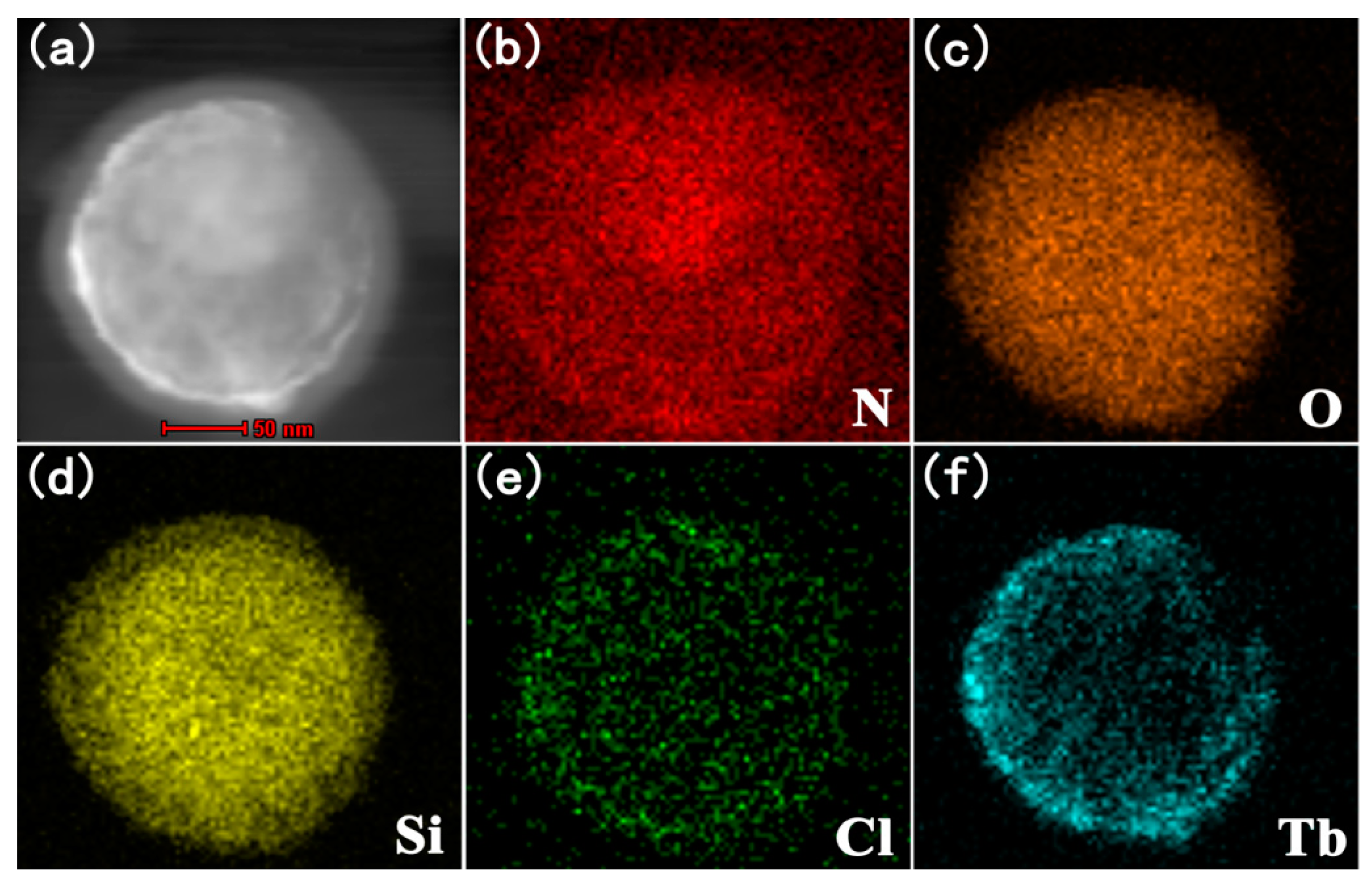
3.2.2. The TEM of SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2
3.3. XPS Analysis
3.4. Thermogravimetric Analysis
3.5. Infrared Spectra Analysis
3.5.1. The FT-IR Spectra of SiO2@PMDA-Si-Tb and SiO2@PMDA-Si-Tb@SiO2
3.5.2. The FT-IR Spectra of SiO2@PMDA-Si-Tb-phen and SiO2@PMDA-Si-Tb-phen@SiO2
3.6. X-ray Diffraction Analysis
3.7. The Photoluminescence Properties
3.8. The Photoluminescence Lifetime
3.9. Low-temperature Phosphorescence Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Rathi, A.K.; Nogueira, I.D.; Varma, R.S.; Branco, P.S. Magnetite-supported sulfonic acid: A retrievable nanocatalyst for the Ritter reaction and multicomponent reactions. Green Chem. 2013, 15, 1895–1899. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Bonifácio, V.D.B.; Varma, R.S.; Nogueira, I.D.; Bundaleski, N.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. Magnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst-applications in multicomponent reactions under benign conditions. Green Chem. 2013, 15, 1226–1231. [Google Scholar] [CrossRef]
- Astruc, D.; Daniel, M.-C. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar]
- Purbia, R.; Paria, S. Yolk/shell nanoparticles: Classifications, synthesis, properties, and applications. Nanoscale 2015, 7, 19789–19873. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Du, Z.; Fan, H.; Wang, R. Nanostructure Optimization of Platinum-Based Nanomaterials for Catalytic Applications. Nanomaterials 2018, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesnert, U. Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett. 2005, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, G.B.; Shi, Y.; Zhang, Y.; Sun, X.; Reich, N.O.; Zhao, D.; Stucky, G. Fabrication of Ag@SiO2@Y2O3: Er Nanostructures for Bioimaging: Tuning of the Upconversion Fluorescence with Silver Nanoparticles -- Supporting Info. J. Am. Chem. Soc. 2010, 132, 2850–2851. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Gong, X.; Peng, S.; Wen, W.; Sheng, P.; Li, W. Design and fabrication of magnetically functionalized core/shell microspheres for smart drug delivery. Adv. Funct. Mater. 2009, 19, 292–297. [Google Scholar] [CrossRef]
- Fredin, L.A.; Li, Z.; Ratner, M.A.; Lanagan, M.T.; Marks, T.J. Enhanced energy storage and suppressed dielectric loss in oxide core-shell-polyolefin nanocomposites by moderating internal surface area and increasing shell thickness. Adv. Mater. 2012, 24, 5946–5953. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, L.; Mei, B.; Tu, J.; Wang, R.; Chen, M.; Cheng, Y. A Rapid Surface-Enhanced Raman Scattering (SERS) Method for Pb2+ Detection Using L-Cysteine-Modified Ag-Coated Au Nanoparticles with Core–Shell Nanostructure. Coatings 2018, 8, 394. [Google Scholar] [CrossRef]
- Yin, D.; Wang, C.; Ouyang, J.; Zhang, X.; Jiao, Z.; Feng, Y.; Song, K.; Liu, B.; Cao, X.; Zhang, L.; et al. Synthesis of a novel core-shell nanocomposite Ag@SiO2@Lu2O3:Gd/Yb/Er for large enhancing upconversion luminescence and bioimaging. ACS Appl. Mater. Interf. 2014, 6, 18480–18488. [Google Scholar] [CrossRef] [PubMed]
- Polido Legaria, E.; Saldan, I.; Svedlindh, P.; Wetterskog, E.; Gunnarsson, K.; Kessler, V.G.; Seisenbaeva, G.A. Coordination of rare earth element cations on the surface of silica-derived nanoadsorbents. D. Trans. 2018, 47, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; García De Abajo, F.J. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wan, J.; Shan, Y.; Chen, K.; Han, X. A facile approach to fabrication of bifunctional magnetic-optical Fe3O4@ZnS microspheres. Chem. Mater. 2009, 21, 4892–4898. [Google Scholar] [CrossRef]
- Wang, L.; Clavero, C.; Huba, Z.; Carroll, K.J.; Carpenter, E.E.; Gu, D.; Lukaszew, R.A. Plasmonics and enhanced magneto-optics in core-shell Co-Ag nanoparticles. Nano Lett. 2011, 11, 1237–1240. [Google Scholar] [CrossRef]
- Georgakilas, V.; Bourlinos, A.B.; Zboril, R.; Steriotis, T.A.; Dallas, P.; Stubos, A.K.; Trapalis, C. Organic functionalisation of graphenes. Chem. Commun. 2010, 46, 1766–1768. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Pykal, M.; Šafářová, K.; Šišková, K.M.; Jurečka, P.; Bourlinos, A.B.; Zbořil, R.; Otyepk, M. Lipid enhanced exfoliation for production of graphene nanosheets. J. Phys. Chem. C 2013, 117, 11800–11803. [Google Scholar] [CrossRef]
- Zbořil, R.; Karlický, F.; Bourlinos, A.B.; Steriotis, T.A.; Stubos, A.K.; Georgakilas, V.; Šafářová, K.; Jančík, D.; Trapalis, C.; Otyepka, M. Graphene fluoride: A stable stoichiometric graphene derivative and its chemical conversion to graphene. Small 2010, 6, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, H.; Wang, F.; Zeng, L. Design of yolk-shell Fe3O4@PMAA composite microspheres for adsorption of metal ions and pH-controlled drug delivery. J. Mater. Chem. A 2014, 2, 7065–7074. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Zou, R.; Gong, S.; Shi, J.; Jiao, J.; Wong, K.L.; Zhang, H.; Wang, J.; Su, Q. Magnetic-NIR Persistent Luminescent Dual-Modal ZGOCS@MSNs@Gd2O3 Core-Shell Nanoprobes for in Vivo Imaging. Chem. Mater. 2017, 29, 3938–3946. [Google Scholar] [CrossRef]
- Peng, D.L.; Hihara, T.; Sumiyama, K.; Morikawa, H. Structural and magnetic characteristics of monodispersed Fe and oxide-coated Fe cluster assemblies. J. Appl. Phys. 2002, 92, 3075–3083. [Google Scholar] [CrossRef]
- She, H.; Chen, Y.; Chen, X.; Zhang, K.; Wang, Z.; Peng, D.L. Structure, optical and magnetic properties of Ni@Au and Au@Ni nanoparticles synthesized via non-aqueous approaches. J. Mater. Chem. 2012, 22, 2757–2765. [Google Scholar] [CrossRef]
- Park, H.H.; Woo, K.; Ahn, J.P. Core-shell bimetallic nanoparticles robustly fixed on the outermost surface of magnetic silica microspheres. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined nanospaces in metallocages: Guest molecules, weakly encapsulated anions, and catalyst sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Feng, Y.; Chen, H. Exploiting core-shell synergy for nanosynthesis and mechanistic investigation. Acc. Chem. Res. 2013, 46, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, X.; Li, S.; Chen, M.; Lu, H.; Yang, Y. Ag@SiO2 Core-shell Nanoparticles Embedded in a TiO2 Mesoporous Layer Substantially Improve the Performance of Perovskite Solar Cells. Nanomaterials 2018, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Kukułka, W.; Wenelska, K.; Baca, M.; Chen, X.; Mijowska, E. From Hollow to Solid Carbon Spheres: Time-Dependent Facile Synthesis. Nanomaterials 2018, 8, 861. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Douvalis, A.P.; Zboril, R.; Bourlinos, A.B.; Tucek, J.; Spyridi, S.; Bakas, T. A facile synthetic route toward air-stable magnetic nanoalloys with Fe-Ni/Fe-Co core and iron oxide shell. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef]
- Maity, D.; Zoppellaro, G.; Sedenkova, V.; Tucek, J.; Safarova, K.; Polakova, K.; Tomankova, K.; Diwoky, C.; Stollberger, R.; MacHala, L.; et al. Surface design of core-shell superparamagnetic iron oxide nanoparticles drives record relaxivity values in functional MRI contrast agents. Chem. Commun. 2012, 48, 11398–11400. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, L.; Han, W.; Xin, X.; Lin, Z. A general and robust strategy for the synthesis of nearly monodisperse colloidal nanocrystals. Nat. Nanotechnol. 2013, 8, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, J.U.; Choi, S.; Son, J.; Oh, M. Synthesis and photoluminescence properties of Eu3+-doped silica@coordination polymer core-shell structures and their calcinated silica@Gd2O3:Eu and hollow Gd2O3:Eu microsphere products. Small 2013, 9, 561–569. [Google Scholar] [CrossRef]
- Tseng, T.K.; Choi, J.; Davidson, M.; Holloway, P.H. Synthesis and luminescent characteristics of europium dopants in SiO2/Gd2O3 core/shell scintillating nanoparticles. J. Mater. Chem. 2010, 20, 6111–6115. [Google Scholar] [CrossRef]
- Shibata, H.; Imakita, K.; Fujii, M. Fabrication of a core-shell-shell particle with a quarter-wave thick shell and its optical properties. RSC Adv. 2014, 4, 32293–32297. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Lehn, J.-M. Luminescent lanthanide complexes as photochemical supramolecular devices. Coord. Chem. Rev. 1993, 123, 201–228. [Google Scholar] [CrossRef]
- Yang, K.S.; Li, Y.L.; Ma, Y.Y.; Feng, L.N.; Wu, A.P.; Qiao, Y.; Bao, J.R.; Li, W.X.; Zhu, X.W.; Yu, R.B. Synthesis and photoluminescence properties of novel core–shell–shell SiO2@CePO4:Tb@SiO2 submicro-spheres. CrystEngComm 2018, 20, 6351–6357. [Google Scholar] [CrossRef]
- Li, W.X.; Zheng, Y.S.; Cao, X.F.; Bai, J.; Fu, Z.F.; Bao, J.R.; Li, Y.L. Preparation, characterization, and luminescence properties of dysprosium perchlorate with MABA-Si and phen or dipy complexes as well as SiO2@Dy(MABA-Si)L core-shell structure nanometermeter luminescent composites. J. Lumin. 2016, 178, 470–478. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Zheng, Y.; Bao, J.; Li, Y.; Feng, L.; Yang, K. Preparation, characterization and luminescence properties of core–shell ternary terbium composites SiO2(600)@Tb(MABA-Si)L. R. Soc. Open Sci. 2018, 5, 171655. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.F.; Li, W.X.; Bai, J.; Bao, J.R.; Cao, X.F.; Zheng, Y.S. Synthesis, characterization and luminescence of europium perchlorate with MABA-Si complex and coating structure SiO2@Eu(MABA-Si) luminescence nanoparticles. Luminescence 2017, 32, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A. Silica-modified luminescent LaPO4:Eu@LaPO4@SiO2 core/shell nanorods: Synthesis, structural and luminescent properties. Luminescence 2018, 33, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.; Lehmann, H.; Finsel, M.; Klinke, C.; Weller, H.; Vossmeyer, T. Synthesis and Characterization of Monodisperse Metallodielectric SiO2@Pt@SiO2 Core-Shell-Shell Particles. Langmuir 2016, 32, 848–857. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Li, T.; He, X.; Wang, Z. Surface charge effect on the cellular interaction and cytotoxicity of NaYF4:Yb3+, Er3+@SiO2 nanoparticles. RSC Adv. 2015, 5, 7773–7780. [Google Scholar] [CrossRef]
- Pfeifer, S.; Schwarzer, A.; Schmidt, D.; Brendler, E.; Veith, M.; Kroke, E. Precursors for pyromellit-bridged silica sol-gel hybrid materials. New J. Chem. 2013, 37, 169–180. [Google Scholar] [CrossRef]
- Pfeifer, S.; Brendler, E.; Veith, M.; Kroke, E. Hybrid-coatings derived from pyromellitic acid bridged alkoxy-silylalkyl precursors. J. Sol-Gel Sci. Technol. 2014, 70, 191–202. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ji, G.; Gao, X.; Zheng, T.; Guan, W.; Liu, H.; Liu, Z. Postsynthetic Metalation Metal-Organic Framework as a Fluorescent Probe for the Ultrasensitive and Reversible Detection of PO43- Ions. Inorg. Chem. 2018, 57, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Q. An efficient optical-electrochemical dual probe for highly sensitive recognition of dopamine based on terbium complex functionalized reduced graphene oxide. Nanoscale 2014, 6, 4583–4587. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Brites, C.D.S.; Bao, S.S.; Ferreira, R.A.S.; Zheng, L.M.; Carlos, L.D. A cryogenic luminescent ratiometric thermometer based on a lanthanide phosphonate dimer. J. Mater. Chem. C 2015, 3, 8480–8484. [Google Scholar] [CrossRef]
- Liu, K.; You, H.; Zheng, Y.; Jia, G.; Song, Y.; Huang, Y.; Yang, M.; Jia, J.; Guo, N.; Zhang, H. Facile and rapid fabrication of metal-organic framework nanobelts and color-tunable photoluminescence properties. J. Mater. Chem. 2010, 20, 3272–3279. [Google Scholar] [CrossRef]
- Ladol, J.; Khajuria, H.; Sheikh, H.N.; Khajuria, Y. Synthesis and characterization of bi-functional magneto-luminescent Fe3O4@SiO2@NaLuF4:Eu3+ hybrid core/shell nanospheres. J. Chem. Sci. 2016, 128, 1149–1155. [Google Scholar] [CrossRef]
- Ansari, A.A.; Joselito, P.L.; Aslam Manthrammel, M. Synthesis, structural, and photoluminescence studies of LaF3:Pr, LaF3:Pr@LaF3, and LaF3:Pr@LaF3@SiO2 nanophosphors. J. Aus. Ceram. Soc. 2018, 54, 493–500. [Google Scholar] [CrossRef]









| Composites | Slit Width (nm) | λEX (nm) | λEM (nm) | I (a.u.) | Intensity Changes |
|---|---|---|---|---|---|
| SiO2@PMDA-Si-Tb | 1.0 | 312 | 543 | 2,040,000 | - |
| SiO2@PMDA-Si-Tb@SiO2 | 1.0 | 322 | 543 | 7,420,000 | 3.64 |
| SiO2@PMDA-Si-Tb-phen | 1.0 | 339 | 543 | 3,730,000 | - |
| SiO2@PMDA-Si-Tb-phen@SiO2 | 1.0 | 330 | 543 | 7,314,118 | 1.96 |
| Composites | A1 | A2 | τ1 (µs) | τ2 (µs) | τ (µs) | R2 |
|---|---|---|---|---|---|---|
| SiO2@PMDA-Si-Tb | 620.558 | 2817.895 | 409.1 | 975.2 | 927.3 | 0.999 |
| SiO2@PMDA-Si-Tb-phen | 2844.215 | 1319.362 | 1009.5 | 126.9 | 960.8 | 0.999 |
| SiO2@PMDA-Si-Tb@SiO2 | 598.777 | 1044.710 | 1373.6 | 805.4 | 1086.2 | 0.999 |
| SiO2@PMDA-Si-Tb-phen@SiO2 | 442.512 | 1256.689 | 424.8 | 1048.2 | 970.1 | 0.998 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Li, W.; Bao, J.; Zheng, Y.; Li, Y.; Ma, Y.; Yang, K.; Qiao, Y.; Wu, A. Synthesis and Luminescence Properties of Core-Shell-Shell Composites: SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2. Nanomaterials 2019, 9, 189. https://doi.org/10.3390/nano9020189
Feng L, Li W, Bao J, Zheng Y, Li Y, Ma Y, Yang K, Qiao Y, Wu A. Synthesis and Luminescence Properties of Core-Shell-Shell Composites: SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2. Nanomaterials. 2019; 9(2):189. https://doi.org/10.3390/nano9020189
Chicago/Turabian StyleFeng, Lina, Wenxian Li, Jinrong Bao, Yushan Zheng, Yilian Li, Yangyang Ma, Kuisuo Yang, Yan Qiao, and Anping Wu. 2019. "Synthesis and Luminescence Properties of Core-Shell-Shell Composites: SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2" Nanomaterials 9, no. 2: 189. https://doi.org/10.3390/nano9020189
APA StyleFeng, L., Li, W., Bao, J., Zheng, Y., Li, Y., Ma, Y., Yang, K., Qiao, Y., & Wu, A. (2019). Synthesis and Luminescence Properties of Core-Shell-Shell Composites: SiO2@PMDA-Si-Tb@SiO2 and SiO2@PMDA-Si-Tb-phen@SiO2. Nanomaterials, 9(2), 189. https://doi.org/10.3390/nano9020189





