Investigation on the Crystallization Behaviors of Polyoxymethylene with a Small Amount of Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of POM/TBOP-TFSI Blends
2.3. Characterization
2.3.1. Antistatic Properties
2.3.2. Mechanical Properties
2.3.3. Scanning Electron Microscopy
2.3.4. Fourier Transfrom Infrared (FTIR)
2.3.5. Differential Scanning Calorimetry
2.3.6. Polarized Light Microscopy
3. Results
3.1. Morphologies of POM/TBOP-TFSI Blends and the Interactions between POM and TBOP-TFSI
3.2. Crystallization Behaviors of POM/TBOP-TFSI Blends
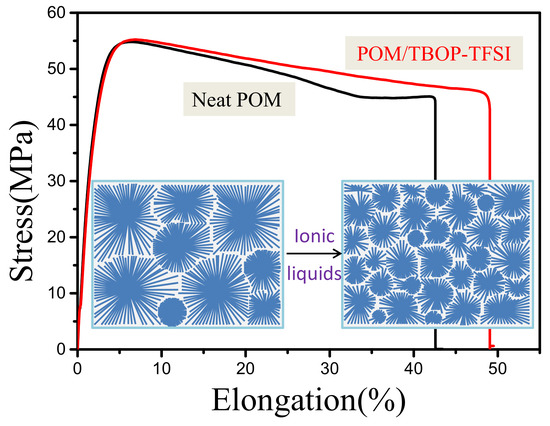
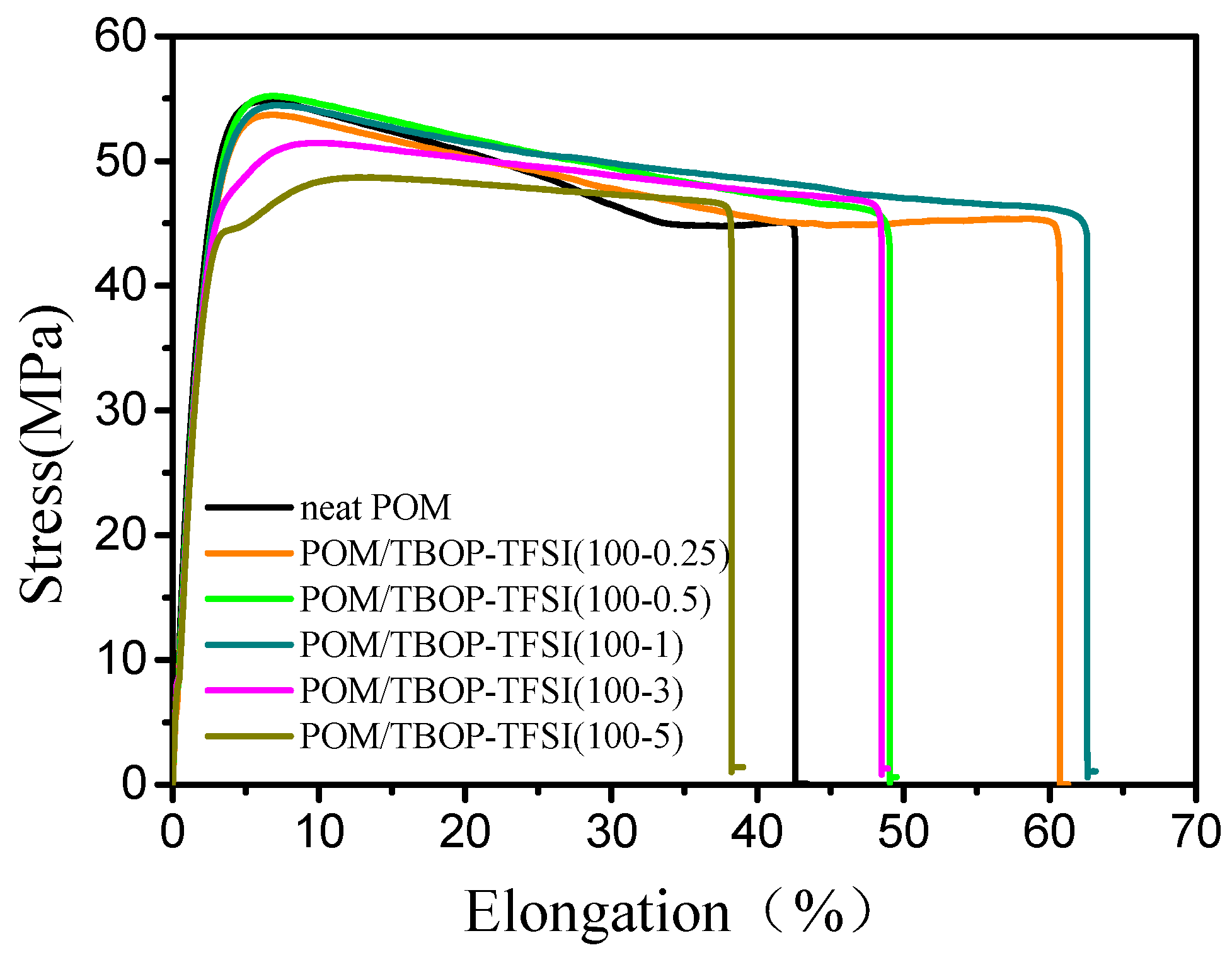
3.3. Mechanical Properties of POM/TBOP-TFSI Blends
3.4. Antistatic Properties of POM/TBOP-TFSI Blends
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hama, H.; Tashiro, K. Structural changes in non-isothermal crystallization process of melt-cooled polyoxymethylene. [I] Detection of infrared bands characteristic of folded and extended chain crystal morphologies and extraction of a lamellar stacking model. Polymer 2003, 44, 3107–3116. [Google Scholar] [CrossRef]
- Hama, H.; Tashiro, K. Structural changes in isothermal crystallization process of polyoxymethylene investigated by time-resolved FTIR, SAXS and WAXS measurements. Polymer 2003, 44, 6973–6988. [Google Scholar] [CrossRef]
- Hasegawa, S.; Takeshita, H.; Yoshii, F.; Sasaki, T.; Makuuchi, K.; Nishimoto, S. Thermal degradation behavior of gamma-irradiated acetyloxy end-capped poly(oxymethylene). Polymer 2000, 41, 111–120. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Y.; Liu, Z. The morphology, crystallization and conductive performance of a polyoxymethylene/carbon nanotube nanocomposite prepared under microinjection molding conditions. J. Polym. Res. 2014, 21, 451. [Google Scholar] [CrossRef]
- Li, K.; Xiang, D.; Lei, X. Green and self-lubricating polyoxymethylene composites filled with low-density polyethylene and rice husk flour. J. Appl. Polym. Sci. 2010, 108, 2778–2786. [Google Scholar] [CrossRef]
- Erol, B.; Ibrahim Unal, H.; Sari, B. Electrorheology and creep-recovery behavior of conducting polythiophene/poly(oxymethylene)-blend suspensions. Chin. J. Polym. Sci. 2012, 30, 16–25. [Google Scholar] [CrossRef]
- Archodoulaki, V.M.; Lüftl, S.; Seidler, S. Thermal degradation behaviour of poly(oxymethylene): 1. Degradation and stabilizer consumption. Polym. Degrad. Stab. 2004, 86, 75–83. [Google Scholar] [CrossRef]
- Lüftl, S.; Archodoulaki, V.M.; Seidler, S. Thermal-oxidative induced degradation behaviour of polyoxymethylene (POM) copolymer detected by TGA/MS. Polym. Degrad. Stab. 2006, 91, 464–471. [Google Scholar] [CrossRef]
- Xu, W.; He, P. Isothermal crystallization behavior of polyoxymethylene with and without nucleating agents. J. Appl. Polym. Sci. 2001, 80, 304–310. [Google Scholar] [CrossRef]
- Bao, H.D.; Guo, Z.X.; Yu, J. Effect of electrically inert particulate filler on electrical resistivity of polymer/multi-walled carbon nanotube composites. Polymer 2008, 49, 3826–3831. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, Q. Morphology control of polyoxy-methylene/thermoplastic polyurethane blends by adjusting their viscosity ratio. Polym. Int. 2010, 55, 1075–1080. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, H.; Zhang, X.; Wu, S.; Xiong, R. Antistatic ability of 1-n-tetradecyl-3-methylimidazolium bromide and its effects on the structure and properties of polypropylene. Eur. Polym. J. 2008, 44, 1247–1251. [Google Scholar] [CrossRef]
- Mehrabzadeh, M.; Rezaie, D. Impact modification of polyacetal by thermoplastic elastomer polyurethane. J. Appl. Polym. Sci. 2010, 84, 2573–2582. [Google Scholar] [CrossRef]
- Pecorini, T.J.; Hertzberg, R.W.; Manson, J.A. Structure-property relations in an injection-moulded, rubber-toughened, semicrystalline polyoxymethylene. J. Mater. Sci. 1990, 25, 3385–3395. [Google Scholar] [CrossRef]
- Pielichowski, K.; Leszczynska, A. Structure-Property Relationships in Polyoxymethylene/Thermoplastic Polyurethane Elastomer Blends: Journal of Polymer Engineering. J. Polym. Eng. 2011, 25, 160–165. [Google Scholar]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Tang, G.; Shao, C.; Hu, X.; Tang, T. The mechanical properties of carbon fiber-reinforced polyoxymethylene composites filled with silaned nano-SiO2. J. Thermoplast. Compos. Mater. 2014, 29, 1020–1029. [Google Scholar] [CrossRef]
- Zhao, X.; Zhen, L.; Jian, L.; Fei, F.Y. The effect of CF and nano-SiO2 modification on the flexural and tribological properties of POM composites. J. Thermoplast. Compos. Mater. 2012, 27, 287–296. [Google Scholar]
- Zhao, X.; Ye, L. Structure and mechanical properties of polyoxymethylene/multi-walled carbon nanotube composites. Compos. Part B 2011, 42, 926–933. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, L. Nucleation effect of polyamide on polyoxymethylene. Polym. Eng. Sci. 2010, 45, 1174–1179. [Google Scholar] [CrossRef]
- Sanes, J.; Carrion, F.; Bermudez, M.D.; Martineznicolas, G. Ionic liquids as lubricants of polystyrene and polyamide 6-steel contacts. Preparation and properties of new polymer-ionic liquid dispersions. Tribol. Lett. 2006, 21, 121–133. [Google Scholar] [CrossRef]
- Li, X.; Qin, A.; Zhao, X.; Ma, B.; He, C. The plasticization mechanism of polyacrylonitrile/1-butyl-3-methylimidazolium chloride system. Polymer 2014, 55, 5773–5780. [Google Scholar] [CrossRef]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic Liquid as Surfactant Agent of Hydrotalcite: Influence on the Final Properties of Polycaprolactone Matrix. Polymer 2018, 10, 44. [Google Scholar] [CrossRef]
- Dou, J.; Liu, Z. Crystallization behavior of poly(ethylene terephthalate)/pyrrolidinium ionic liquid. Polym. Int. 2013, 62, 1698–1710. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, M.; Zhao, L.; You, J.; Cao, X.; Li, Y. Ionic liquid modified poly(vinylidene fluoride): crystalline structures, miscibility, and physical properties. Polym. Chem. 2013, 4, 5726–5734. [Google Scholar] [CrossRef]
- Tsurumaki, A.; Tajima, S.; Iwata, T.; Scrosati, B.; Ohno, H. Antistatic effects of ionic liquids for polyether-based polyurethanes. Electrochim. Acta 2015, 175, 13–17. [Google Scholar] [CrossRef]
- Iwata, T.; Tsurumaki, A.; Tajima, S.; Ohno, H. Fixation of ionic liquids into polyether-based polyurethane films to maintain long-term antistatic properties. Polymer 2014, 55, 2501–2504. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Foksowicz-Flaczyk, J. Ionic liquids with symmetrical dialkoxymethyl-substituted imidazolium cations. Chemistry 2004, 10, 3479–3485. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Tan, J. Preparation of an ionic-liquid antistatic/photostabilization additive and its effects on polypropylene. J. Vinyl Addit. Technol. 2010, 16, 58–63. [Google Scholar] [CrossRef]
- Mejri, R.; Dias, J.C.; Lopes, A.C.; Hentati, S.B.; Silva, M.M.; Botelho, G.; De Ferro, A.M.; Esperanca, J.M.S.S.; Maceiras, A.; Laza, J.M. Effect of ionic liquid anion and cation on the physico-chemical properties of poly(vinylidene fluoride)/ionic liquid blends. Eur. Polym. J. 2015, 71, 304–313. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, J.; Zhao, Y.; Qiu, Y.; Li, J. Room Temperature Ionic Liquid Based Polystyrene Nanofibers with Superhydrophobicity and Conductivity Produced by Electrospinning. Chem. Mater. 2008, 20, 3420–3424. [Google Scholar]
- Wei, T.; Pang, S.; Xu, N.; Pan, L.; Zhang, Z.; Xu, R.; Ma, N.; Lin, Q. Crystallization behavior and isothermal crystallization kinetics of PLLA blended with ionic liquid, 1-butyl-3-methylimidazolium dibutylphosphate. J. Appl. Polym. Sci. 2015, 132, 41308–41319. [Google Scholar] [CrossRef]
- Yu, N.; He, L.; Ren, Y.; Xu, Q. High-crystallization polyoxymethylene modification on carbon nanotubes with assistance of supercritical carbon dioxide: Molecular interactions and their thermal stability. Polymer 2011, 52, 472–480. [Google Scholar] [CrossRef]





| Samples | Tc (°C) | Tm (°C) | Tm–Tc (°C) | t1/2 (min) at 152 °C | χc (%) |
|---|---|---|---|---|---|
| Neat POM | 145.5 | 165.4 | 19.9 | 5.9 | 51.1 |
| POM/TBOP-TFSI (100-0.25) | 145.7 | 164.9 | 19.2 | 6.4 | 51.3 |
| POM/TBOP-TFSI (100-0.5) | 147.1 | 165.2 | 18.1 | 2.4 | 53.2 |
| POM/TBOP-TFSI (100-1) | 146.2 | 165.0 | 18.8 | 5.5 | 53.6 |
| POM/TBOP-TFSI (100-3) | 146.8 | 164.9 | 18.1 | 5.3 | 52.9 |
| POM/TBOP-TFSI (100-5) | 146.8 | 164.8 | 18.0 | 5.3 | 52.0 |
| Sample | Yeilding Strength (MPa) | Elongation at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|
| Neat POM | 55.2 ± 0.8 | 40.2 ± 10 | 4.2 |
| POM/TBOP-TFSI (100-0.25) | 53.8 ± 0.7 | 56.1 ± 11 | 4.6 |
| POM/TBOP-TFSI (100-0.5) | 54.7 ± 0.5 | 47.0 ± 9 | 4.7 |
| POM/TBOP-TFSI (100-1) | 54.2 ± 0.8 | 63.3 ± 12 | 4.5 |
| POM/TBOP-TFSI (100-3) | 51.5 ± 0.5 | 42.0 ± 6 | 4.4 |
| POM/TBOP-TFSI (100-5) | 48.3 ± 1.0 | 39.2 ± 13 | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Q.; Chen, Q.; Wang, L.; Chen, H.; Li, Y. Investigation on the Crystallization Behaviors of Polyoxymethylene with a Small Amount of Ionic Liquid. Nanomaterials 2019, 9, 206. https://doi.org/10.3390/nano9020206
Jiao Q, Chen Q, Wang L, Chen H, Li Y. Investigation on the Crystallization Behaviors of Polyoxymethylene with a Small Amount of Ionic Liquid. Nanomaterials. 2019; 9(2):206. https://doi.org/10.3390/nano9020206
Chicago/Turabian StyleJiao, Qi, Qin Chen, Lian Wang, Hualin Chen, and Yongjin Li. 2019. "Investigation on the Crystallization Behaviors of Polyoxymethylene with a Small Amount of Ionic Liquid" Nanomaterials 9, no. 2: 206. https://doi.org/10.3390/nano9020206