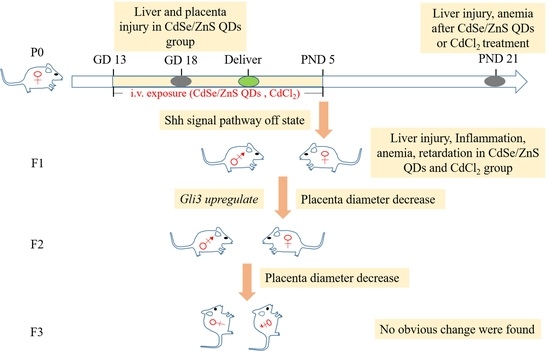
CdSe/ZnS Quantum Dots Impaired the First Two Generations of Placenta Growth in an Animal Model, Based on the Shh Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Animal Treatment
2.2. ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analysis Cd Caontent
2.3. Histopathological Examination
2.4. Hematological Analysis and Serum Biomarkers Assay
2.5. RT-qPCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Characterization of CdSe/ZnS QDs
3.2. Body Weight Changes of Pregnant Mice
3.3. Cd Contents Analysis in Parental Mice (P0)
3.4. Hematology and Serum Biochemistry
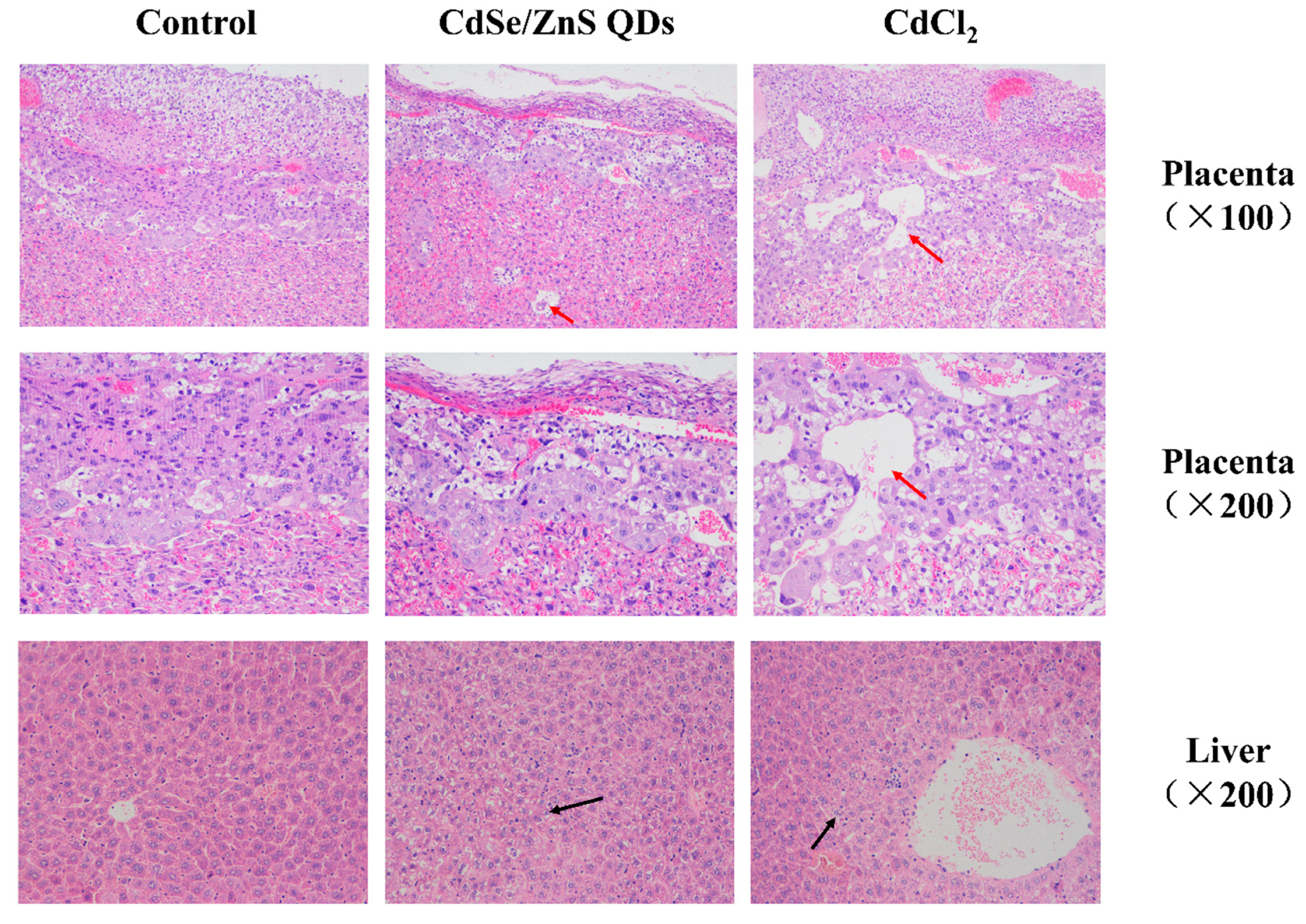
3.5. Histopathological Examination
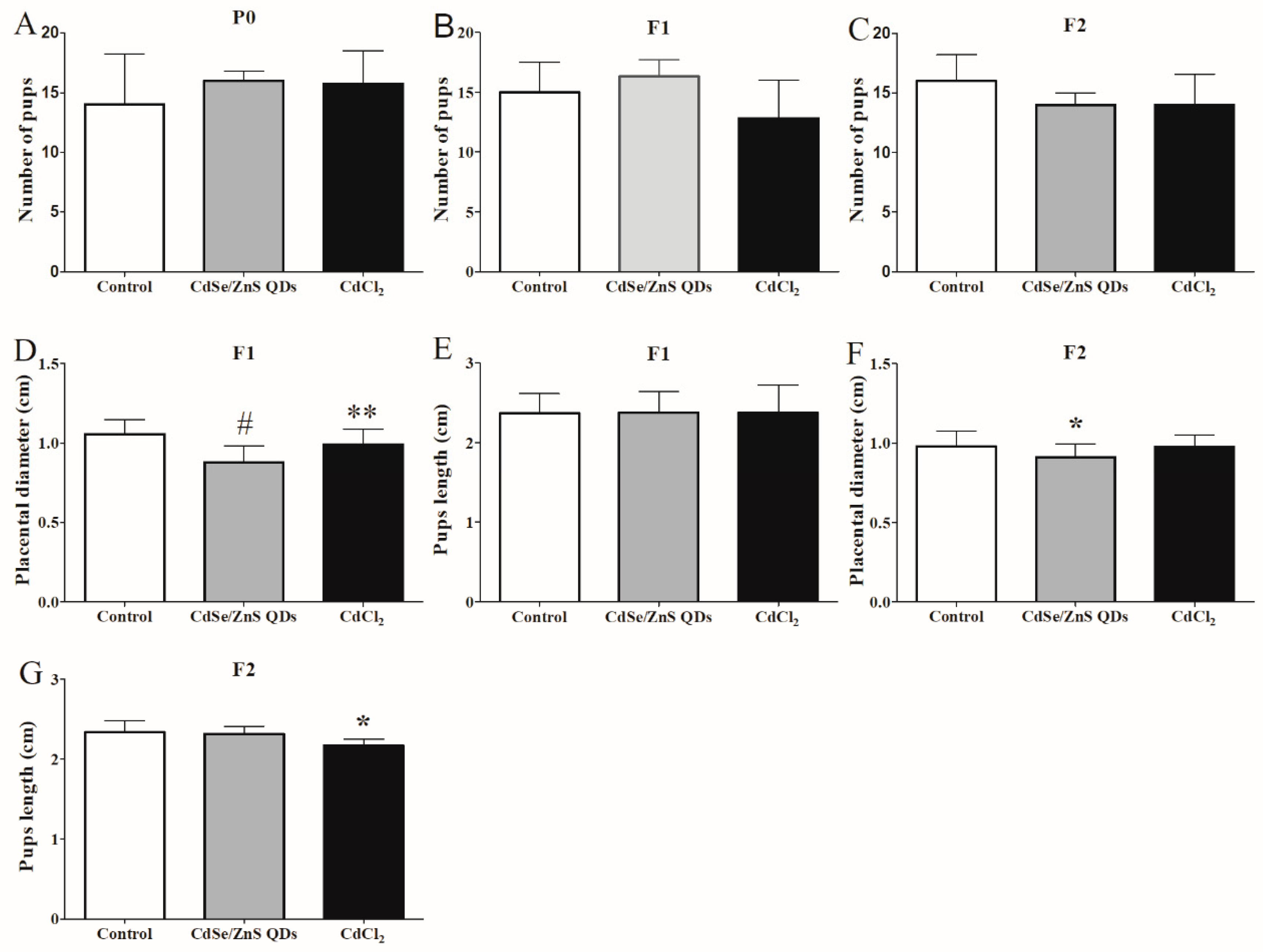
3.6. Offspring Development
3.7. RT-qPCR Analysis
4. Discussion
4.1. QDs Induced Maternal Injury
4.2. QDs Accumulate in the Placenta but Do Not Pass to the Next Generation
4.3. QDs Impaired the First Two Generations of Placenta Growth and Induced Offspring Injury
4.4. QD May Cause Toxicity through Shh Signal Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhu, J.J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilan, R.; Fleury, F.; Nabiev, I.; Sukhanova, A. Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjugate Chem. 2015, 26, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Han, S.L.; Liu, H.M.; Chen, X.; Huang, L.; Li, X.H.; Zhang, J.X. The role of surface chemistry in determining in vivo biodistribution and toxicity of CdSe/ZnS core-shell quantum dots. Biomaterials 2013, 34, 8741–8755. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xu, Y.M.; Wu, D.D.; Yao, Y.; Liang, Z.L.; Tan, H.W.; Lau, A.T. Acute and chronic cadmium telluride quantum dots-exposed human bronchial epithelial cells: The effects of particle sizes on their cytotoxicity and carcinogenicity. Biochem. Bioph. Res. Co. 2018, 495, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yang, L.; Kuang, H.J.; Yang, P.F.; Aguilar, Z.P.; Wang, A.; Fu, F.; Xu, H.Y. Acute toxicity of quantum dots on late pregnancy mice: Effects of nanoscale size and surface coating. J. Hazard. Mater. 2016, 318, 61–69. [Google Scholar] [CrossRef]
- Chu, M.Q.; Wu, Q.W.; Yang, H.; Yuan, R.Q.; Hou, S.K.; Yang, Y.F.; Zou, Y.J.; Xu, S.; Xu, K.Y.; Ji, A.L.; et al. Transfer of quantum dots from pregnant mice to pups across the placental barrier. Small 2010, 6, 670–678. [Google Scholar] [CrossRef]
- Liu, J.W.; Yang, C.B.; Liu, J.; Hu, R.; Hu, Y.Z.; Chen, H.Y.; Law, W.C.; Swihart, M.T.; Ye, L.; Wang, K.; et al. Effects of cd-based quantum dot exposure on the reproduction and offspring of kunming mice over multiple generations. Nanotheranostics 2017, 1, 23–37. [Google Scholar] [CrossRef]
- Ma, J.; Li, R.B.; Liu, Y.; Qu, G.B.; Liu, J.; Guo, W.L.; Song, H.Y.; Li, X.H.; Liu, Y.J.; Xia, T.; et al. Carbon nanotubes disrupt iron homeostasis and induce anemia of inflammation through inflammatory pathway as a secondary effect distant to their portal-of-entry. Small 2017, 13, 1603830. [Google Scholar] [CrossRef] [PubMed]
- Zalgeviciene, V.; Kulvietis, V.; Bulotiene, D.; Zurauskas, E.; Laurinaviciene, A.; Skripka, A.; Rotomskis, R. Quantum dots mediated embryotoxicity via placental damage. Reprod. Toxicol. 2017, 73, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.J.; Crompton, L.A.; Sood, A.; Saunders, M.; Boyle, N.T.; Buckley, A.; Minogue, A.M.; Mccomish, S.F.; Jiménezmoreno, N.; Corderollana, O. Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes. Nat. Nanotechnol. 2018, 13, 427–433. [Google Scholar] [CrossRef]
- Zhang, G.B.; Wang, H.; Hu, J.; Guo, M.Y.; Wang, Y.; Zhou, Y.; Yu, Z.; Fu, L.; Chen, Y.H.; Xu, D.X. Cadmium-induced neural tube defects and fetal growth restriction: Association with disturbance of placental folate transport. Toxicol. Appl. Pharm. 2016, 306, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Barański, B. Behavioral alterations in offspring of female rats repeatedly exposed to cadmium oxide by inhalation. Toxicol. Lett. 1984, 22, 53–61. [Google Scholar] [CrossRef]
- Adeyemo, O.K. Haematological profile of Clarias gariepinus (Burchell, 1822) exposed to lead. Turk. J. Fish Aquat. Sci. 2007, 1, 163–169. [Google Scholar]
- Giannini, E.; Botta, F.; Fasoli, A.; Ceppa, P.; Risso, D.; Lantieri, P.B.; Celle, G.; Testa, R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Digest. Dis. Sci. 1999, 44, 1249–1253. [Google Scholar] [CrossRef]
- Pak, E.; Segal, R.A. Hedgehog Signal Transduction: Key Players, Oncogenic Drivers, and Cancer Therapy. Dev. Cell 2016, 38, 333–344. [Google Scholar] [CrossRef]
- Bellusci, S.; Furuta, Y.; Rush, M.G.; Henderson, R.; Winnier, G.; Hogan, B. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 1997, 124, 53–63. [Google Scholar]
- Chen, J.K.; Taipale, J.; Cooper, M.K.; Beachy, P.A. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002, 16, 2743–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kugler, M.C.; Joyner, A.L.; Loomis, C.A.; Munger, J.S. Sonic hedgehog signaling in the lung. From development to disease. Am. J. Resp. Cell Mol. 2015, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rajat, R.; Ljiljana, M.; Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007, 317, 372–376. [Google Scholar]
- Mo, R.; Freer, A.M.; Zinyk, D.L.; Crackower, M.A.; Michaud, J.; Heng, H.H.; Chik, K.W.; Shi, X.M.; Tsui, L.C.; Cheng, S.H.; et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 1997, 124, 113–123. [Google Scholar] [PubMed]
- Park, H.L.; Bai, C.; Platt, K.A.; Matise, M.P.; Beeghly, A.; Hui, C.C.; Nakashima, M.; Joyner, A.L. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 2000, 127, 1593–1605. [Google Scholar] [PubMed]
- Litingtung, Y.; Dahn, R.D.; Li, Y.; Fallon, J.F.; Chiang, C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 2002, 418, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.B.; Gong, Y.; Ruan, H.F.; Pan, L.Y.; Wu, X.K.; Tang, C.; Wang, C.J.; Zhu, H.B.; Zhang, Z.M.; Tang, L.F. Sonic hedgehog through Gli2 and Gli3 is required for the proper development of placental labyrinth. Cell Death Dis. 2015, 6, e1653. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Mazar, J.; Neal, C.J.; Rosado, A.; Das, S.; Westmoreland, T.; Seal, S. Nanoparticle delivery of curcumin induces cellular hypoxia and ROS-mediated apoptosis via modulation of Bcl-2/Bax in human neuroblastoma. Nanoscale 2017, 9, 10375–10387. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czabotar, P.E.; Guillaume, L.; Andreas, S.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Alam, J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Resp. Cell Mol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.P.; Magilnick, N.; Lee, C.; Kalmaz, D.; Ou, X.P.; Chan, J.Y.; Lu, S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-κB and AP-1. Mol. Cell. Biol. 2005, 25, 5933–5946. [Google Scholar] [CrossRef] [PubMed]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme oxygenases in cardiovascular health and disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef] [PubMed]
- Korge, P.; Calmettes, G.; Weiss, J.N. Increased reactive oxygen species production during reductive stress: the roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta 2015, 1847, 514–525. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, W.; Kuang, H.; He, X.; Yang, L.; Yang, P.; Chen, B.; Aguilar, Z.P.; Xu, H. CdSe/ZnS Quantum Dots Impaired the First Two Generations of Placenta Growth in an Animal Model, Based on the Shh Signaling Pathway. Nanomaterials 2019, 9, 257. https://doi.org/10.3390/nano9020257
Hong W, Kuang H, He X, Yang L, Yang P, Chen B, Aguilar ZP, Xu H. CdSe/ZnS Quantum Dots Impaired the First Two Generations of Placenta Growth in an Animal Model, Based on the Shh Signaling Pathway. Nanomaterials. 2019; 9(2):257. https://doi.org/10.3390/nano9020257
Chicago/Turabian StyleHong, Wuding, Huijuan Kuang, Xingping He, Lin Yang, Pengfei Yang, Bolu Chen, Zoraida P. Aguilar, and Hengyi Xu. 2019. "CdSe/ZnS Quantum Dots Impaired the First Two Generations of Placenta Growth in an Animal Model, Based on the Shh Signaling Pathway" Nanomaterials 9, no. 2: 257. https://doi.org/10.3390/nano9020257