Role of Structure and Composition on the Performances of P-Type Tin Oxide Thin-Film Transistors Processed at Low-Temperatures
Abstract
1. Introduction
2. Materials, Methods and Results
2.1. Experimental Details
Films Preparation
2.2. Structure Morphology, Composition and Electro-Optical Data and Analysis
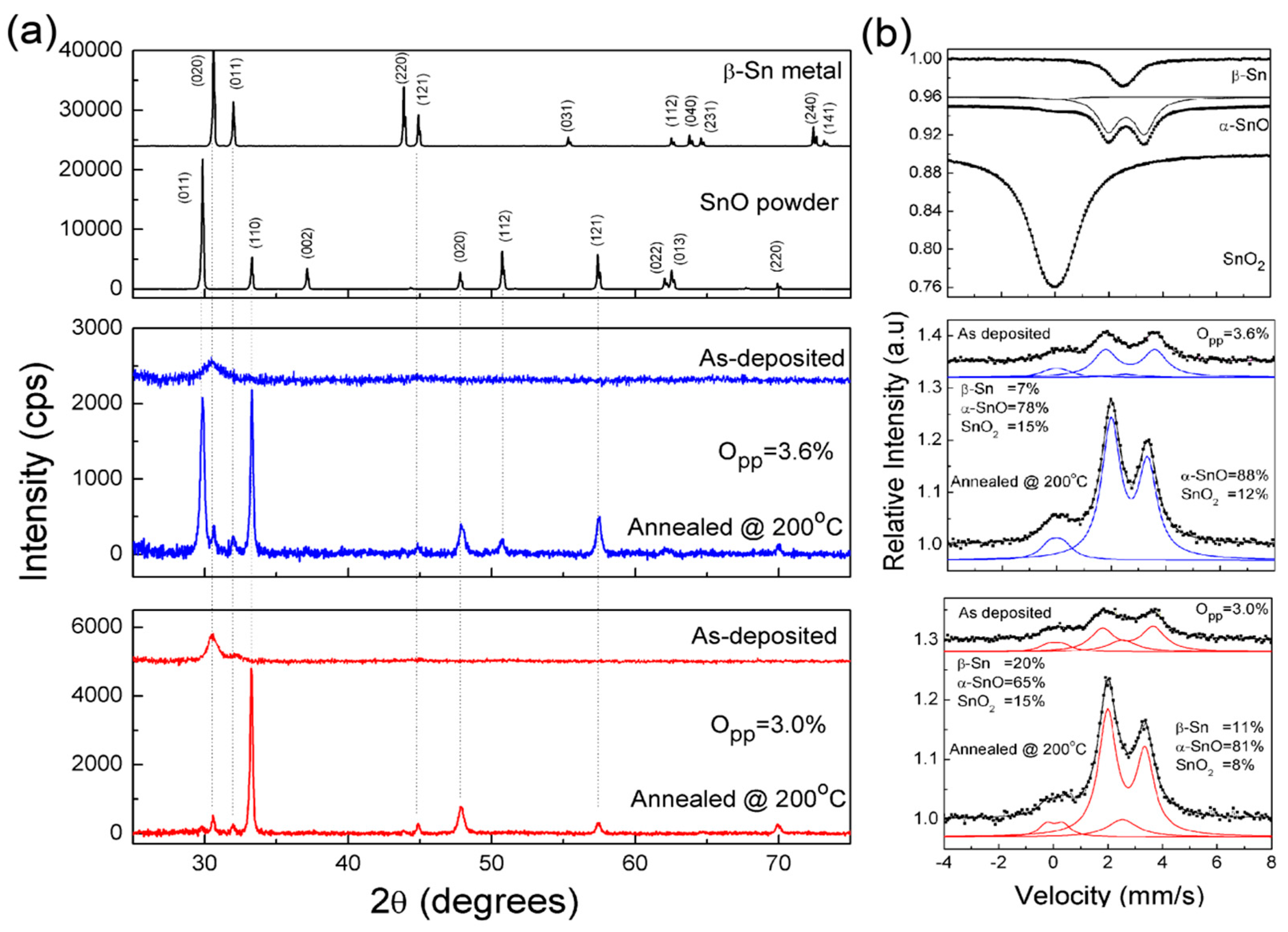
2.3. Structure Data and Analysis
2.4. Electrical Data and Analysis
2.5. Optical Data and Analysis
3. Devices Results and Analysis
3.1. Devices Structure, Geometry, Fabrication and Characterization Conditions
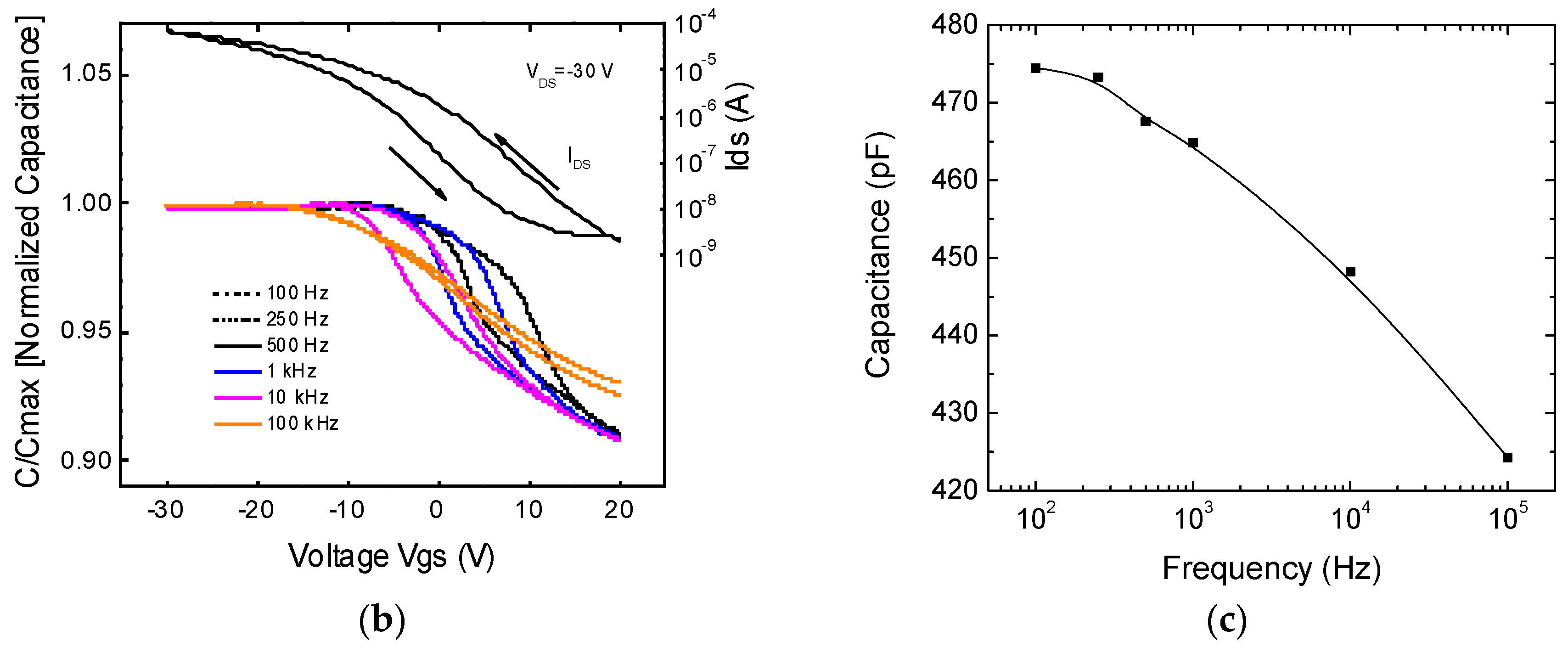
3.2. Capacitance Measurements Data and Analysis
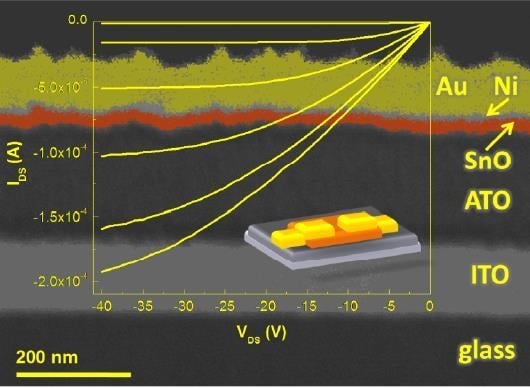
3.3. TFT Electrical Data and Analysis
4. Discussion of the Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klasens, H.A.; Koelmans, H. A Tin oxide Field-Effect Transistor. Solid-State Electron. 1964, 7, 701–702. [Google Scholar] [CrossRef]
- Boesen, G.F.; Jacobs, J.E. ZnO Field Effect Transistor. Proc. IEEE 1968, 56, 2094. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 435, 488. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.L.; Norris, B.J.; Wager, J.F. ZnO-based transparent thin-film transistors. Appl. Phys. Lett. 2003, 82, 733. [Google Scholar] [CrossRef]
- Carcia, P.F.; McLean, R.S.; Reilly, M.H.; Nunes, G. Transparent ZnO thin-film transistor fabricated by rf magnetron sputtering. Appl. Phys. Lett. 2003, 82, 1117. [Google Scholar]
- Fortunato, E.; Barquinha, P.; Pimental, A.; Goncalves, A.; Marques, A.; Pereira, L.; Martins, R. Fully transparent ZnO thin-film transistor produced at room temperature. Adv. Mater. 2005, 17, 590. [Google Scholar] [CrossRef]
- Martins, R.; Ferreira, I.; Fortunato, E. Electronics with and on paper. Phys. Status Solidi-RRL 2011, 5, 332–335. [Google Scholar] [CrossRef]
- Fortunato, E.; Martins, R. Where science fiction meets reality? With oxide semiconductors! Phys. Status Solidi-RRL 2011, 5, 336. [Google Scholar] [CrossRef]
- Senanayak, S.P.; Sangwan, V.K.; McMorrow, J.J.; Everaerts, K.; Chen, Z.H.; Facchetti, A.; Hersam, M.C.; Marks, T.J.; Narayan, K.S. Self-Assembled Photochromic Molecular Dipoles for High-Performance Polymer Thin-Film Transistors. ACS Appl. Mater. Interfaces 2018, 10, 21492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.W.; Lee, E.K.; Park, J.I.; Lee, B.L.; Kwon, Y.N.; Byun, S.; Jung, M.S.; Kim, J.J. Cross-linked poly(hydroxy imide) gate-insulating materials for low-temperature processing of organic thin-film transistors. J. Mater. C 2018, 6, 13359–13366. [Google Scholar] [CrossRef]
- Street, R.A. Thin-Film Transistors. Adv. Mater. 2009, 21, 2007. [Google Scholar] [CrossRef]
- Dimitrakopoulos, C.D.; Malenfant, P.R.L. Organic thin film transistors for large area electronics. Adv. Mater. 2002, 14, 99. [Google Scholar] [CrossRef]
- Chen, F.Z.; Jiang, Y.; Sui, Y.; Zhang, J.D.; Tian, H.K.; Han, Y.; Deng, Y.F.; Hu, W.P.; Geng, Y.H. Donor-Acceptor Conjugated Polymers Based on Bisisoindigo: Energy Level Modulation toward Unipolar n-Type Semiconductors. Macromolecules 2018, 51, 8652–8661. [Google Scholar] [CrossRef]
- Kumagai, S.; Nakano, M.; Takimiya, K.; Takeya, J. Solution-crystallized n-type organic thin-film transistors: An impact of branched alkyl chain on high electron mobility and thermal durability. Org. Electron. 2018, 62, 548–553. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Santa, A.; Santos, A.; Bahubalindruni, P.; Deuermeier, J.; Martins, R.; Fortunato, E.; Barquinha, P. A Sustainable Approach to Flexible Electronics with Zinc-Tin Oxide Thin-Film Transistors. Adv. Elecron. Mater. 2018, 4, 1800032. [Google Scholar] [CrossRef]
- Fortin, E.; Weichman, F.L. Hall effect and electrical conductivity of Cu2O monocrystals. Can. J. Phys. 1966, 44, 1551. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Nomura, K.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. Epitaxial growth of high mobility Cu(2)O thin films and application to p-channel thin film transistor. Appl. Phys. Lett. 2008, 93, 202107. [Google Scholar] [CrossRef]
- Deuermeier, J.; Fortunato, E.; Martins, R.; Klein, A. Energy band alignment at the nanoscale. Appl. Phys. Lett. 2017, 110, 051603. [Google Scholar] [CrossRef]
- Liu, A.; Nie, S.B.; Liu, G.X.; Zhu, H.H.; Zhu, C.D.; Shin, B.; Fortunato, E.; Martins, R.; Shan, F. In situ one-step synthesis of p-type copper oxide for low-temperature, solution-processed thin-film transistors. J. Master. Chem. C 2017, 5, 2524. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.H.; Guo, Z.D.; Meng, Y.; Liu, G.X.; Fortunato, E.; Martins, R.; Shan, F.K. Solution Combustion Synthesis: Low-Temperature Processing for p-Type Cu:NiO Thin Films for Transparent Electronics. Adv. Mater. 2017, 29, 1701599. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.K.; Liu, A.; Zhu, H.H.; Kong, W.J.; Liu, J.Q.; Shin, B.C.; Fortunato, E.; Martins, R.; Liu, G.X. High-mobility p-type NiOx thin-film transistors processed at low temperatures with Al2O3 high-k dielectric. J. Mater. Chem. C 2016, 4, 9438–9444. [Google Scholar] [CrossRef]
- Stedile, F.C.; Debarros, B.A.S.; Leite, C.B.; Freire, F.L.; Baumvol, I.J.R.; Schreiner, W.H. Characterization of Tin Oxide Thin Films depopsited by reactive sputtering. Thin Solid Films 1989, 170, 285–291. [Google Scholar] [CrossRef]
- Togo, A.; Oba, F.; Tanaka, I.; Tatsumi, K. First-principles calculations of native defects in tin monoxide. Phys. Rev. B 2006, 74, 195128. [Google Scholar] [CrossRef]
- Allen, J.P.; Scanlon, D.O.; Parker, S.C.; Watson, G.W. Tin Monoxide: Structural Prediction from First Principles Calculations with van der Waals Corrections. J. Phys. Chem. C 2011, 115, 19916. [Google Scholar] [CrossRef]
- Pan, X.Q.; Fu, L. Tin oxide thin films grown on the ((1)over-bar012) sapphire substrate. J. Electrocer. 2001, 7, 35. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47. [Google Scholar] [CrossRef]
- Fortunato, E.; Barros, R.; Barquinha, P.; Figueiredo, V.; Park, S.H.; Hwang, C.S.; Martins, R. Transparent p-type SnOx thin film transistors produced by reactive rf magnetron sputtering followed by low temperature annealing. Appl. Phys. Lett. 2010, 97, 052105. [Google Scholar] [CrossRef]
- Caraveo-Frescas, J.A.; Jesus, A.; Nayak, P.K.; Al-Jawhari, H.A.; Granato, D.B.; Schwingenschlogl, U.; Alshareeft, H.N. Record Mobility in Transparent p-Type Tin Monoxide Films and Devices by Phase Engineering. ACS Nano 2013, 7, 5160. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.W.; Lin, H.C.; Liu, K.C.; Huang, T.Y. Improving Electrical Performances of p-Type SnO Thin-Film Transistors Using Double-Gated Structure. IEEE Electron. Device Lett. 2015, 36, 1053–1055. [Google Scholar] [CrossRef]
- Shih, C.W.; Chin, A.; Lu, C.F.; Su, W.F. Remarkably High Hole Mobility Metal-Oxide Thin-Film Transistors. Sci. Rep. 2018, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, H.; Kaji, N.; Hayashi, R.; Kumomi, H.; Nomura, K.; Kamiya, T.; Hirano, M.; Hosono, H. Sputtering formation of p-type SnO thin-film transistors on glass toward oxide complimentary circuits. Appl. Phys. Lett. 2010, 97, 072111. [Google Scholar] [CrossRef]
- Martins, R.; Nathan, A.; Barros, R.; Pereira, L.; Barquinha, P.; Correia, N.; Costa, R.; Ahnood, A.; I Ferreira, I.; Fortunato, E. Complementary Metal Oxide Semiconductor Technology with and on Paper. Adv. Mater. 2011, 23, 4491. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Chen, W.-C.; Tsai, Y.-T.; Kung, Y.-C.; Chang, C.-H.; Hsu, C.-J.; Wu, C.-C.; Hsieh, H.-H. Fabrication of p-Type SnO Thin-Film Transistors by Sputtering with Practical Metal Electrodes. Jpn. J. Appl. Phys. 2013, 52, 05DC07. [Google Scholar] [CrossRef]
- Chiu, I.C.; Li, Y.-S.; Tu, M.-S.; Cheng, I.C. Complementary Oxide–Semiconductor-Based Circuits With n-Channel ZnO and p-Channel SnO Thin-Film Transistors. IEEE Electron Device Lett. 2014, 35, 1263. [Google Scholar] [CrossRef]
- Myeonghun, U.; Han, Y.; Song, S.; Cho, I.; Lee, J.; Kwon, H.-I. High Performance p-type SnO thin-film Transistor with SiOx Gate Insulator Deposited by Low-Temperature PECVD Method. J. Semicond. Technol. Sci. 2014, 14, 666. [Google Scholar]
- Caraveo-Frescas, J.A.; Khan, M.A.; Alshareef, H.N. Polymer ferroelectric field-effect memory device with SnO channel layer exhibits record hole mobility. Sci. Rep. 2014, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liang, L.Y.; Liu, Q.; Cao, H.T. Magnetron-Sputtered SnO Thin Films for p-Type and Ambipolar TFT Applications. ECS J. Solid State Sci. Technol. 2014, 3, Q3091. [Google Scholar] [CrossRef]
- Khan, M.A.; Caraveo-Frescas, J.A.; Alshareef, H.N. Hybrid dual gate ferroelectric memory for multilevel information storage. Org. Electron. 2015, 16, 9. [Google Scholar] [CrossRef]
- Han, Y.; Choi, Y.; Jeong, H.; Kwon, H.I. Investigation of Intrinsic Electrical Characteristics and Contact Effects in p-Type Tin Monoxide Thin-Film Transistors Using Gated-Four-Probe Measurements. J. Nanosci. Nanotechnol. 2015, 15, 7582. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Choi, Y.J.; Jeong, C.Y.; Lee, D.; Song, S.H.; Kwon, H.I. Environment-Dependent Bias Stress Stability of P-Type SnO Thin-Film Transistors. IEEE Electron Device Lett. 2015, 36, 466. [Google Scholar] [CrossRef]
- Saji, K.J.; Mary, A.P.R. Tin Oxide Based P and N-Type Thin Film Transistors Developed by RF Sputtering. ECS J. Solid State Sci. Technol. 2015, 4, Q101. [Google Scholar] [CrossRef]
- Hsu, P.C.; Tsai, S.P.; Chang, C.H.; Hsu, C.J.; Chen, W.C.; Hsieh, H.H.; Wu, C.C. Preparation of p-type SnO thin films and transistors by sputtering with robust Sn/SnO2 mixed target in hydrogen-containing atmosphere. Thin Solid Films 2015, 585, 50. [Google Scholar] [CrossRef]
- Zhong, C.-W.; Lin, H.-C.; Tsai, J.-R.; Liu, J.-R.; Huang, T.-Y. Impact of gate dielectrics and oxygen annealing on tin-oxide thin-film transistors. Jpn. J. Appl. Phys. 2016, 55, 04EG02. [Google Scholar] [CrossRef]
- Bae, S.; Kwon, S.-H.; Jeong, H.; Kwon, H. Demonstration of high-performance p-type tin oxide thin-film transistors using argon-plasma surface treatments. Semicond. Sci. Technol. 2017, 32, 075006. [Google Scholar] [CrossRef]
- Yang, V.; Wang, Y.; Li, Y.; Yuan, Y.; Hu, Z.; Ma, P.; Zhou, L.; Wang, Q.; Song, A.; Xin, Q. Highly Optimized Complementary Inverters Based on p-SnO and n-InGaZnO With High Uniformity. IEEE Electron Device Lett. 2018, 39, 516. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Z.; Hota, M.K.; Alshareef, H.N.; Wu, T. P-Type SnO Thin Film Phototransistor with Perovskite-Mediated Photogating. Adv. Electron. Mater. 2019, 1, 1800538. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, J.; Li, Y.; Zhang, J.; Wang, Q.; Song, A.; Xin, Q. Organic and inorganic passivation of p-type SnO thin-film transistors with different active layer thicknesses. Semicond. Sci. Technol. 2018, 33, 075001. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Yang, J.; Yuan, Y.; Hu, Z.; Lin, Z.; Song, A.; Xin, Q. Complementary Integrated Circuits Based on n-Type and p-Type Oxide Semiconductors for Applications Beyond Flat-Panel Displays. IEEE Trans. Electron Devices 2019, 66, 950. [Google Scholar] [CrossRef]
- Ogo, Y.; Hiramatsu, H.; Nomura, K.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. p-channel thin-film transistor using p-type oxide semiconductor, SnO. Appl. Phys. Lett. 2008, 93, 032113. [Google Scholar]
- Nomura, K.; Kamiya, T.; Hosono, H. Ambipolar Oxide Thin-Film Transistor. Adv. Mater. 2011, 23, 3431. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Wu, C.-C.; Hiramatsu, H.; Kamiya, T.; Hosono, H. Film Texture, Hole Transport and Field-Effect Mobility in Polycrystalline SnO Thin Films on Glass. ECS J. Solid State Sci. Technol. 2014, 3, Q3040. [Google Scholar] [CrossRef]
- Liang, L.; Cao, H. Ambipolar SnO thin-film transistors and inverters. ECS Trans. 2012, 50, 289. [Google Scholar] [CrossRef]
- Hung, M.P.; Genoe, J.; Heremans, P.; Steudel, S. Off-current reduction in p-type SnO thin film transistors. Appl. Phys. Lett. 2018, 112, 2635021. [Google Scholar]
- Chen, P.-C.; Chiu, Y.-C.; Zheng, Z.-W.; Cheng, C.-H.; Wu, Y.-H. P-type tin-oxide thin film transistors for blue-light detection application. Phys. Status Solidi-Rapid Res. Lett. 2016, 10, 919. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chiu, Y.-C.; Zheng, Z.-W.; Cheng, C.-H.; Wu, Y.-H. Influence of plasma fluorination on p-type channel tin-oxide thin film transistors. J. Alloys Compd. 2017, 707, 162. [Google Scholar] [CrossRef]
- Kim, S.K.H.; Baek, I.-H.; Kim, D.H.; Pyeon, J.J.; Chung, T.-M.; Baek, S.-H.; Kim, J.-S.; Han, J.H.; Kim, S.K. Fabrication of high-performance p-type thin film transistors using atomic-layer-deposited SnO films. J. Mater. Chem. C 2017, 5, 3139. [Google Scholar] [CrossRef]
- Okamura, K.; Nasr, B.; Brand, R.A.; Hahn, H. Solution-processed oxide semiconductor SnO in p-channel thin-film transistors. J. Mater. Chem. 2012, 22, 4607. [Google Scholar] [CrossRef]
- Shenoy, G.K.; Wagner, F.E. Mössbauer Isomer Shifts; North Holland Publ. Co.: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Blanca, E.; Svane, A.; Christensen, N.E.; Rodriguez, C.O.; Cappannini, O.M.; Moreno, M.S. Calculated Static and dynamic Properties of beta-Sn and Sn-O compounds. Phys. Rev. B 1993, 48, 15712. [Google Scholar] [CrossRef]
- Conte, D.E.; Aboulaich, A.; Robert, F.; Olivier-Fourcade, J.; Jumas, J.C.; Jordy, C.; Willmann, P. Sn-x[BPO4](1-x) composites as negative electrodes for lithium ion cells: Comparison with amorphous SnB0.6P0.4O2.9 and effect of composition. J. Solid State Chem. 2010, 183, 65. [Google Scholar] [CrossRef]
- Herber, R.H. Mössbauer Lattice temperature of Tetragonal (P4NMM) SnO. Phys. Rev. B 1983, 27, 4013. [Google Scholar] [CrossRef]
- Moreno, M.S.; Mercader, R.C. Mössbauer study of SnO Lattice dynamics. Phys. Rev. B 1994, 50, 9875. [Google Scholar] [CrossRef]
- Hosono, H.; Ogo, Y.; Yanagi, H.; Kamiya, T. Bipolar Conduction in SnO Thin Films. Electrochem. Solid State Lett. 2011, 14, II13. [Google Scholar] [CrossRef]
- Jeon, S.; Ahn, S.E.; Song, I.; Kim, C.J.; Chung, U.I.; Lee, E.; Yoo, I.; Nathan, A.; Lee, S.; Robertson, J.; et al. Gated three-terminal device architecture to eliminate persistent photoconductivity in oxide semiconductor photosensor arrays. Nat. Mater. 2012, 11, 301–305. [Google Scholar] [CrossRef] [PubMed]







| Method | Device Structure | Process Temp. (°C) | Opp (%) | Dielectric | μh,FE (cm2 V−1 s−1) | Ion/Ioff | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| RFMS | SBG | RT * | 11.5 | ATO | 1.2 | 103 | 2010 | [28] |
| RFMS | SBG | 300 | 1 | SiNx | 0.24 | 102 | 2010 | [32] |
| RFMS | SBG | 150 | n.r. | Paper | 1.3 | 102 | 2011 | [33] |
| RFMS | SBG | 250 | n.r. | SiO2 | 1.8 | 103 | 2013 | [34] |
| RFMS | SBG | 225 | 4.3 | HfO2 | 0.33 | 103 | 2014 | [35] |
| RFMS | SBG | 230 | n.r. | SiO2 | 0.59 | 3 × 103 | 2014 | [36] |
| RFMS | CTG | 200 | 9 | P(VDF-TrFE) | 3.3 | 3 × 102 | 2014 | [37] |
| RFMS | SBG | 200 | 11.8 | SiO2 | 1.36 | 2 × 103 | 2014 | [38] |
| RFMS | STG | 200 | 9 | P(VDF-TrFE) | 2.7 | 2 × 102 | 2014 | [39] |
| RFMS | SBG | 200 | n.r. | SiO2 | 0.61 | 6.2 × 105 | 2015 | [40] |
| RFMS | SBG | 250 | n.r. | SiO2 | 1.8 | 105 | 2015 | [41] |
| RFMS | SBG | 200 | 7.5 | SiO2 | 4.13 | 6 × 102 | 2015 | [42] |
| RFMS | SBG | 250 | - | SiO2 | 1.16 | 2.3 × 102 | 2015 | [43] |
| RFMS | SBG | 300 | n.r. | SiO2 | 3.33 | 104 | 2016 | [44] |
| RFMS | SBG | 200 | n.r. | SiO2 | 0.63 | 5.2 × 106 | 2017 | [45] |
| RFMS | SBG | 225 | n.r. | Al2O3 | 0.7 | 2.6 × 104 | 2018 | [46] |
| RFMS | SBG | 120 | 9 | HfO2 | 5.53 | 2.7 × 103 | 2018 | [47] |
| RFMS | SBG | 225 | 3.1 | SiO2 | 1.41 | 1.5 × 103 | 2018 | [48] |
| RFMS | SBG | 225 | 3.1 | SiO2 | 0.87 | 1.88 × 104 | 2019 | [49] |
| RFMS | SBG | RT * | 3.0 | ATO | 4.6 | 7 × 104 | 2019 | This work |
| Method | Device Structure | Process Temp. (°C) | Oxygen Partial Pressure (%) | Dielectric | μh,FE (cm2 V−1 s−1) | Ion/Ioff | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| PLD | STG | 575 | 4 × 10−2 Pa | Al2O3 | 1.3 | 102 | 2008 | [50] |
| PLD | STG | 250 | 1 × 10−2 Pa | SiO2 | 0.81 | ~102 | 2011 | [51] |
| PLD | SBG | 300 | 1 × 10−2 Pa | SiO2 | 2.18 | -- | 2014 | [52] |
| EBE | SBG | 400 | n.r. | SiO2 | 0.32 | 5 × 102 | 2013 | [53] |
| TE | SBG | 250 | n.r. | Al2O3 | 1.4 | 5 × 104 | 2018 | [54] |
| DCMS | SBG | 180 | 9 | HfO2 | 6.75 | ~103 | 2013 | [29] |
| DCMS | DG | 300 | 3.07 × 10−2 Pa | SiO2 | 6.54 | 105 | 2015 | [30] |
| DCMS | SBG | 200 | n.r. | HfO2 | 5.56 | 4.8 × 104 | 2016 | [55] |
| DCMS | SBG | 200 | n.r. | HfO2 | 7.6 | 3 × 104 | 2018 | [31] |
| PVD | SBG | 200 | n.r. | HfO2 | 2.13 | 9.6 × 106 | 2017 | [56] |
| ALD | SBG | 250 | n.r. | Al2O3 | 1 | 2 × 106 | 2017 | [57] |
| SC | SBG | 450 | - | SiO2 | 0.13 | 85 | 2012 | [58] |
| Sample | IS (mm/s) | QS (mm/s) | Γ (mm/s) | Sn Phase | I (%) |
|---|---|---|---|---|---|
| SnO2 bulk | 0.01 | 0.56 | 1.34 | - | 100 |
| α-SnO bulk | 2.67 −0.03 | 1.34 0.58 | 0.98 0.77 | α-SnO SnO2 | 95 5 |
| -Sn metal | 2.56 | - | 1.03 | - | 100 |
| Film Opp = 3.0%, RT | 2.76 0.11 2.56 | 1.87 0.52 − | 1.04 0.84 1.3 | SnO SnO2 -Sn | 65 15 20 |
| Film Opp = 3.0%, 200 °C, 30 min | 2.7 0.08 2.56 | 1.38 0.59 − | 0.79 0.7 1.35 | SnO SnO2 -Sn | 81 8 11 |
| Film Opp = 3.6%, RT | 2.73 0.11 2.56 | 1.82 0.53 − | 1.02 0.81 1.3 | SnO SnO2 -Sn | 78 15 7 |
| Film Opp = 3.6%, 200 °C, 30 min | 2.7 0.06 | 1.34 0.48 | 0.82 0.85 | SnO SnO2 | 88 12 |
| Annealing Conditions | Oxygen Partial Pressure (%) | Field Effect Mobility (cm2 V−1 s−1) | Saturation Mobility (cm2 V−1 s−1) | Threshold Voltage (V) | On-Off Ratio | Dominant Composition Phase |
|---|---|---|---|---|---|---|
| 200 °C, 30 min | 3.0 | 3.3 | 4.6 | −10 | 7 × 104 | α-SnO |
| 200 °C, 60 min | 3.0 | 2.2 | 2.6 | −7.2 | 9 × 104 | α-SnO |
| 200 °C, 30 min | 3.6 | 0.16 | 0.28 | −29 | 2 × 105 | α-SnO + β-SnO |
| 200 °C, 60 min | 3.6 | 0.02 | 0.03 | −38.6 | 3 × 104 | α-SnO + β-SnO |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, R.; Saji, K.J.; Waerenborgh, J.C.; Barquinha, P.; Pereira, L.; Carlos, E.; Martins, R.; Fortunato, E. Role of Structure and Composition on the Performances of P-Type Tin Oxide Thin-Film Transistors Processed at Low-Temperatures. Nanomaterials 2019, 9, 320. https://doi.org/10.3390/nano9030320
Barros R, Saji KJ, Waerenborgh JC, Barquinha P, Pereira L, Carlos E, Martins R, Fortunato E. Role of Structure and Composition on the Performances of P-Type Tin Oxide Thin-Film Transistors Processed at Low-Temperatures. Nanomaterials. 2019; 9(3):320. https://doi.org/10.3390/nano9030320
Chicago/Turabian StyleBarros, Raquel, Kachirayil J. Saji, João C. Waerenborgh, Pedro Barquinha, Luís Pereira, Emanuel Carlos, Rodrigo Martins, and Elvira Fortunato. 2019. "Role of Structure and Composition on the Performances of P-Type Tin Oxide Thin-Film Transistors Processed at Low-Temperatures" Nanomaterials 9, no. 3: 320. https://doi.org/10.3390/nano9030320
APA StyleBarros, R., Saji, K. J., Waerenborgh, J. C., Barquinha, P., Pereira, L., Carlos, E., Martins, R., & Fortunato, E. (2019). Role of Structure and Composition on the Performances of P-Type Tin Oxide Thin-Film Transistors Processed at Low-Temperatures. Nanomaterials, 9(3), 320. https://doi.org/10.3390/nano9030320