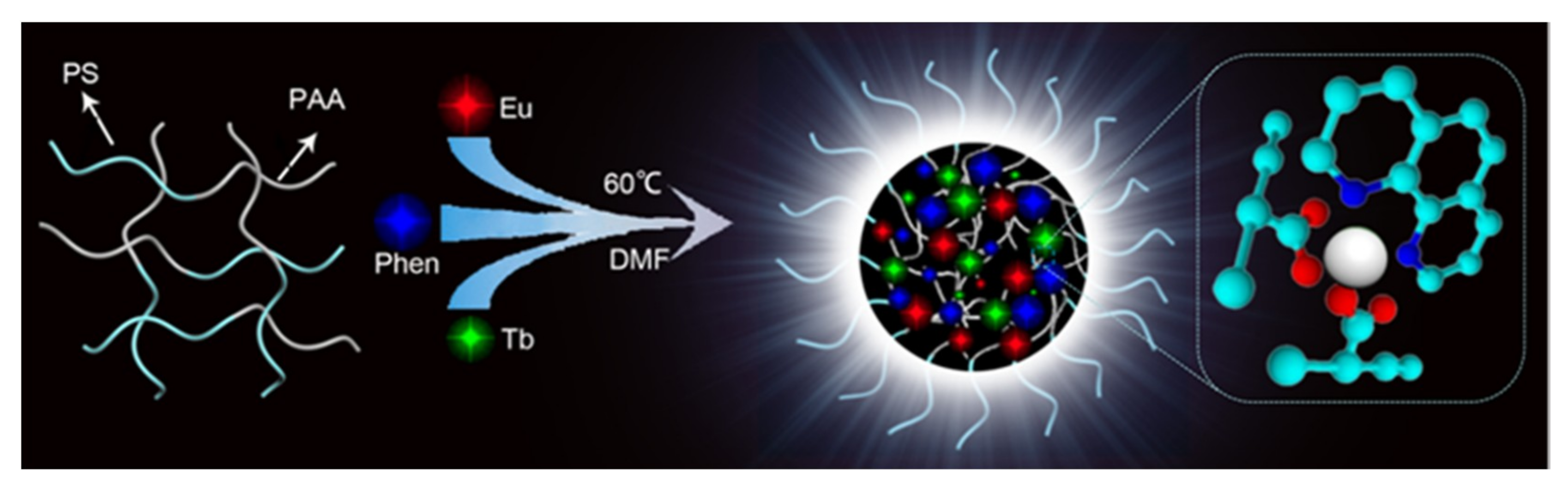
Ln3+-Induced Diblock Copolymeric Aggregates for Fully Flexible Tunable White-Light Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Preparation of Diblock Copolymers: Polystyrene-Block-Polyacrylic Acid (PS-b-PAA)
2.3.1. Synthesis of RAFT Agent
2.3.2. Preparation of Diblock Copolymers PS-b-PAA
2.3.3. Synthesis of Eu3+/Tb3+ Complexes
(1) Synthesis of EuCl3·6H2O and TbCl3·6H2O
(2) Synthesis of Eu3+/Tb3+ Complexes
3. Results and Discussion
3.1. The Structure of Polymeric Aggregates
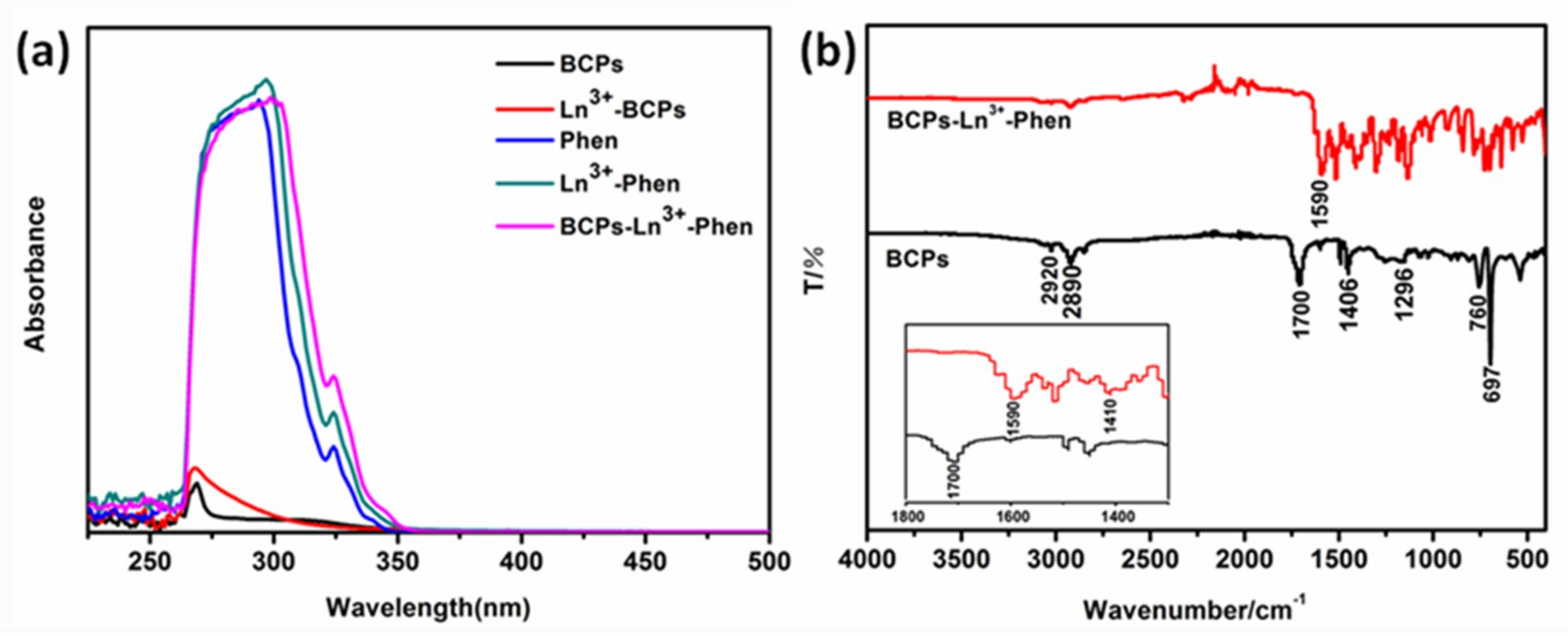
 O [33,34]. These two bonds connected on the same carbon atom have similar vibration frequencies, which can form two absorption peaks at 1590 and 1410 cm−1 ascribed to the occurrence of vibration coupling and asymmetric vibration and symmetric vibration of C
O [33,34]. These two bonds connected on the same carbon atom have similar vibration frequencies, which can form two absorption peaks at 1590 and 1410 cm−1 ascribed to the occurrence of vibration coupling and asymmetric vibration and symmetric vibration of C  O, respectively. In general, the spectrum indicates that the O atom of the carboxyl groups has coordinated with Ln3+ ions.
O, respectively. In general, the spectrum indicates that the O atom of the carboxyl groups has coordinated with Ln3+ ions.3.1.1. X-ray Photoelectron Spectroscopy (XPS)
3.2. Morphologies of Polymeric Assemblies.
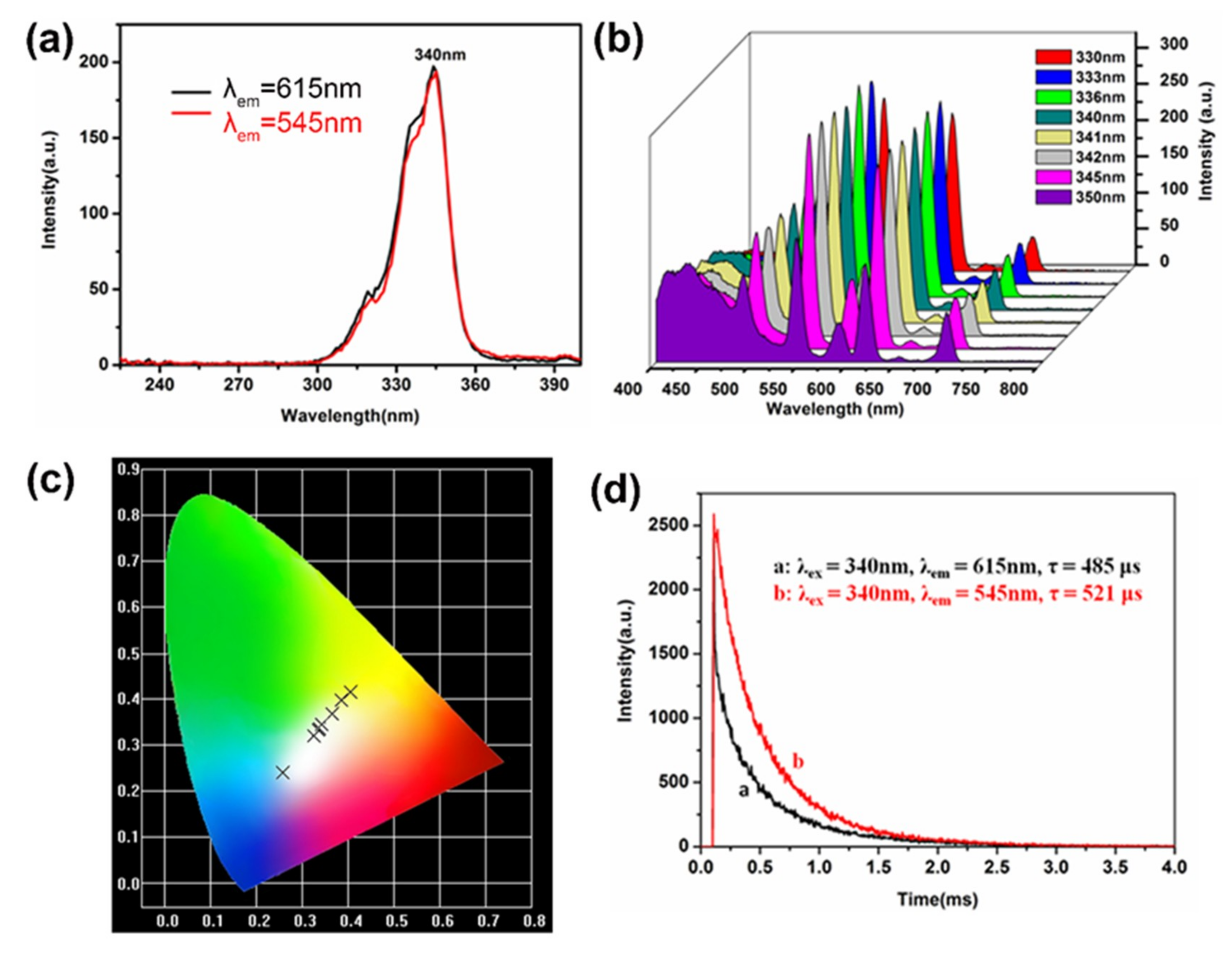
3.3. Fluorescence Property and White Light Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- An, R.; Zhao, H.; Hu, H.M.; Wang, X.; Yang, M.L.; Xue, G. Synthesis, structure, white-Light emission, and temperature recognition properties of Eu/Tb mixed coordination polymers. Inorg. Chem. 2016, 55, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, S.R.; Du, D.Y.; Qin, J.S.; Bao, S.I.; Li, J.; Su, Z.M.; He, W.W.; Fu, Q.; Lan, Y.Q. Stable luminescent metal−organic frameworks as dual-functional materials to encapsulate Ln3+ ions for white-light emission and to detect nitroaromatic explosives. Inorg. Chem. 2015, 54, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yang, Y.; Zhai, G.; Jia, H.; Xu, B. Tuning the chromaticity of the emission color of the copolymers containing Eu (III), Tb (III), Be (II) ions based on colorimetric principle. Opt. Mater. 2016, 52, 92–99. [Google Scholar] [CrossRef]
- Karlicek, R.F. Smart lighting – beyond simple illumination. In Proceedings of the 2012 IEEE Photonics Society Summer Topical Meeting Series, Seattle, WA, USA, 9–11 July 2012; pp. 147–148. [Google Scholar]
- Sanderson, S.W.; Simons, K.L. Light emitting diodes and the lighting revolution: The emergence of a solid-state lighting industry. Res. Policy 2014, 43, 1730–1746. [Google Scholar] [CrossRef]
- Pan, M.; Yan, C.; Chen, L.; Zhang, L.Y.; Yin, S.Y.; Zhu, Y.X.; Wu, K.; Hou, Y.J.; Su, C.Y. Photoluminescence and white-light emission in two series of heteronuclear Pb (II)–Ln (III) complexes. New J. Chem. 2015, 39, 3770–3776. [Google Scholar] [CrossRef]
- Feng, C.; Sun, J.W.; Yan, P.F.; Li, Y.X.; Liu, T.Q.; Sun, Q.Y.; Li, G.M. Color-tunable and white-light emission of one-dimensional L-di-2-thenoyltartaric acid mixed-lanthanide coordination polymers. Dalton Trans. 2015, 44, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Q.; Grindy, S.; Holtenandersen, N. White-light-emitting lanthanide metallogels with tunable luminescence and reversible stimuli-responsive properties. J. Am. Chem. Soc. 2015, 137, 11590–11593. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhuang, W.; Ye, H.; Wang, D.; Zhang, S.; Huang, X. A novel red phosphor for white light emitting diodes. J. Alloys Compd. 2005, 390, 226–229. [Google Scholar] [CrossRef]
- Ravindran, E.; Somanathan, N. Efficient white-light emission from a single polymer system with “spring-like” self-assemblies induced emission enhancement and intramolecular charge transfer characteristics. J. Mater. Chem. C 2017, 5, 4763–4774. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, W.L.; Yang, W.J.; Wang, H.Y. Epitaxial growth of group III-nitride films by pulsed laser deposition and their use in the development of LED devices. Surf. Sci. Rep. 2015, 70, 380–423. [Google Scholar] [CrossRef]
- Bai, G.; Tsang, M.K.; Hao, J. Luminescent ions in advanced composite materials for multifunctional applications. Adv. Fun. Mater. 2016, 26, 6330–6350. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Stan, C.S.; Horlescu, P.; Popa, M.; Coroaba, A.; Ursu, L.E. Photoluminescent polymer composites with R, G, B emission and their potential applications in LCD displays. New J. Chem. 2016, 40, 6505–6512. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, A.; Wei, X. Structure and photoluminescence property of Eu, Tb, Zn-containing macromolecular complex for white light emission. Opt. Laser Technol. 2018, 107, 389–397. [Google Scholar]
- He, D.F.; Tang, Q.; Liu, S.M.; Luo, F.; Liu, Y.W.; Li, N.; Miao, J.; Wang, X.Q.; Chen, X.G.; Ma, F.J. White-light emission by selectively encapsulating single lanthanide metal ions into alkaline earth metal-organic coordination polymers. Dyes Pigment. 2015, 122, 317–323. [Google Scholar] [CrossRef]
- Narayana, Y.S.L.V.; Basak, S.; Baumgarten, M.; Müllen, K.; Chandrasekar, R. White-Emitting Conjugated Polymer/Inorganic Hybrid Spheres: Phenylethynyl and 2, 6-Bis (pyrazolyl) pyridine Copolymer Coordinated to Eu (tta)3. Adv. Funct. Mater. 2013, 23, 5875–5880. [Google Scholar] [CrossRef]
- Kłonkowski, A.M.; Lis, S.; Pietraszkiewicz, M.; Hnatejko, Z.; Czarnobaj, K.; Elbanowski, M. Luminescence properties of materials with Eu (III) complexes: Role of ligand, coligand, anion, and matrix. Chem. Mater. 2003, 15, 656–663. [Google Scholar] [CrossRef]
- Liu, L.; Fu, G.; Li, B.; Lü, X.; Wong, W.; Jones, R.A. Single-component Eu3+–Tb3+–Gd3+-grafted polymer with ultra-high color rendering index white-light emission. RSC Adv. 2017, 7, 6762–6771. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Y.N.; Liu, L.; Lü, X.Q.; Zhu, X.J.; Wong, W.K.; Pan, M.; Su, C.Y. Pure white-light and colour-tuning of Eu3+–Gd3+-containing metallopolymer. Chem. Commun. 2016, 52, 3713–3716. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Li, L.; Yang, Y.; Zhao, X.; Zhang, A.; Jia, H.; Liu, X.; Xu, B. Tunable white light emission of Eu, Tb, Zn-containing copolymers by RAFT polymerization. J. Mater. Chem. C 2015, 3, 9933–9941. [Google Scholar]
- Xiao, Y.; Wang, S.; Zheng, F.; Wu, M.; Xu, J.; Liu, Z.; Chen, J.; Li, R.; Guo, G. Excitation wavelength-induced color-tunable and white-light emissions in lanthanide (III) coordination polymers constructed using an environment-dependent luminescent tetrazolate–dicarboxylate ligand. CrystEngComm 2016, 18, 721–727. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Wagner, T.; Thiel, J.; Yao, Y.; Lin, Y.; Metwalli, E.; Liu, R.; Butt, H.J.; Müllerbuschbaum, P. Solvothermal synthesis of hierarchical Eu2O3 nanostructures templated by PS-b-PMAA: Morphology control via simple variation of water contents. J. Mater. Chem. A 2015, 3, 5789–5793. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, J.; Wang, Y.; Liu, J.; Wang, X.; Huang, Z.; Huang, L.; Wang, Y.; Shen, W.; Belfiore, L.A. Eu3+-induced aggregates of diblock copolymers and their photoluminescent property. J. Colloid Interface Sci. 2013, 394, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S. Synthesis of well-defined homopolymer and diblock copolymer grafted onto silica particles by Z-supported RAFT polymerization. Macromolecules 2006, 39, 8603–8608. [Google Scholar]
- Fréalsaison, S.; Save, M.; Bui, C.; Charleux, B.; Magnet, S. Emulsifier-free controlled free-radical emulsion polymerization of styrene via RAFT using dibenzyltrithiocarbonate as a chain transfer agent and acrylic acid as an ionogenic comonomer: Batch and spontaneous phase inversion processes. Macromolecules 2006, 39, 8632–8638. [Google Scholar] [CrossRef]
- Liu, J.; Setijadi, E.; Liu, Y.; Whittaker, M.R.; Boyer, C.; Davis, T.P. PEGylated Gold Nanoparticles Functionalized with β-Cyclodextrin Inclusion Complexes: Towards Metal Nanoparticle–Polymer–Carbohydrate Cluster Biohybrid Materials. Aust. J. Chem. 2010, 63, 1245–1250. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Feng, W.X.; Yin, S.Y.; Pan, M.; Wang, H.P.; Fan, Y.N.; Lü, X.Q.; Su, C.Y. PMMA-copolymerized color tunable and pure white-light emitting Eu3+-Tb3+ containing Ln-metallopolymers. J. Mater. Chem. C 2017, 5, 1742–1750. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, D.; Don, T. Preparation and photoluminescence properties of polymer−rare-earth complexes composed of bidentate schiff-base-ligand-functionalized polysulfone and Eu (III) ion. J. Phys. Chem. C 2015, 119, 16403–16413. [Google Scholar] [CrossRef]
- Gao, C.; Kirillov, A.M.; Dou, W.; Tang, X.; Liu, L.; Yan, X.; Xie, Y.; Zang, P.; Liu, W.; Tang, Y. Self-assembly synthesis, structural features, and photophysical properties of dilanthanide complexes derived from a novel amide type ligand: Energy transfer from Tb (III) to Eu (III) in a heterodinuclear derivative. Inorg. Chem. 2014, 53, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Divya, V.; Reddy, M.L.P. Visible-light excited red emitting luminescent nanocomposites derived from Eu3+-phenathrene-based fluorinated b-diketonate complexes and multi-walled carbon nanotubes. J. Mater. Chem. C 2013, 1, 160–170. [Google Scholar] [CrossRef]
- Xu, J.; Huang, X.H.; Zhou, N.L.; Zhang, J.S.; Bao, J.C.; Lu, T.H.; Li, C. Synthesis, XPS and fluorescence properties of Eu3+ complex with polydimethylsiloxane. Mater. Lett. 2004, 58, 1938–1942. [Google Scholar] [CrossRef]
- Sun, J.G.; Arunkumar, P.; Im, W.B. A new blue-emitting oxohalide phosphor Sr4OCl6:Eu2+ for thermally stable, efficient white-light-emitting devices under near-UV. J. Phys. Chem. C 2014, 118, 2686–2692. [Google Scholar]
- Liu, L.; Li, H.; Su, P.; Zhang, Z.; Fu, G.; Li, B.; Lü, X. Red to white polymer light-emitting diode (PLED) based on Eu3+–Zn2+–Gd3+-containing metallopolymer. J. Mater. Chem. C 2017, 5, 4780–4787. [Google Scholar] [CrossRef]
- Willis-Fox, N.; Kraft, M.; Arlt, J.; Scherf, U.; Evans, R.C. Tunable white-light emission from conjugated polymer-di-ureasil materials. Adv. Funct. Mater. 2016, 26, 532–542. [Google Scholar] [CrossRef]
- Haldar, D.; Ghosh, A.; Bose, S. Defect induced photoluminescence in MoS2 quantum dots and effect of Eu3+/Tb3+ co-doping towards efficient white light emission. Opt. Mater. 2018, 79, 12–20. [Google Scholar] [CrossRef]
- Yahiaoui, Z.; Hassairi, M.A.; Dammak, M. Tunable luminescence and near white-light emission of YPO4:Eu3+, Tb3+, Tm3+, phosphors. J. Alloys Compd. 2018, 763, 56–61. [Google Scholar] [CrossRef]






| Measurement | Characteristics of Equipment |
|---|---|
| 1H NMR spectra | JNM-ECP600 (600 MHz) spectrometer JEOL Ltd, Kyoto, Japan, CDCl3 as the solvent |
| UV/vis spectra | UV755B spectrophotometer, Youke, Shanghai, China at room temperature |
| NIR spectra | infrared spectrometer, Nicolet 5700, Thermo Fisher Scientific, Varian, Inc., Palo Alto, CA, USA |
| XPS spectra | Kratos spectrometer, XSAM-800, ESCALAB, London, UK |
| TEM micrographs | JEM-1200EX electron microscope, JEOL Ltd., Kyoto, Japan |
| EDS mapping | JEM-2100 high-resolution transmission electron microscope, JEOL, Kyoto, Japan |
| Fluorescent spectra | fluorescence spectrophotometer (Varian, Inc., Palo Alto, CA, USA) at ambient temperature |
| TG curves | Thermo gravimetric analysis, SII TG/DTA 6300, Waltham, MA, USA |
| Lifetime | Fluorescence Spectrometer, FLS980, Edinburgh, UK |
| Samples | Elements | |||
|---|---|---|---|---|
| C (Atomic%) | O (Atomic%) | Eu (Atomic%) | Tb (Atomic%) | |
| a | 86.02 | 13.35 | 0.45 | 0.19 |
| b | 87.01 | 12.70 | 0.19 | 0.10 |
| c | 77.68 | 22.10 | 0.13 | 0.009 |
| d | 92.48 | 7.14 | 0.11 | 0.27 |
| e | 88.85 | 10.70 | 0.11 | 0.33 |
| Samples | Decomposition Temperature (°C) |
|---|---|
| BCPs | 192 |
| BCPs-Ln3+-Phen | 240 |
| Excitation Wavelength (nm) | CIE Coordinates |
|---|---|
| 330 | (0.404, 0.416) |
| 333 | (0.386, 0.396) |
| 336 | (0.364, 0.368) |
| 340 | (0.341, 0.338) |
| 341 | (0.337, 0.334) |
| 342 | (0.335, 0.332) |
| 345 | (0.324, 0.320) |
| 350 | (0.258, 0.239) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tang, J.; Wang, G.; Wang, W.; Ren, J.; Ding, W.; Zhang, X.; Wang, Y.; Shen, W.; Huang, L.; et al. Ln3+-Induced Diblock Copolymeric Aggregates for Fully Flexible Tunable White-Light Materials. Nanomaterials 2019, 9, 363. https://doi.org/10.3390/nano9030363
Wang X, Tang J, Wang G, Wang W, Ren J, Ding W, Zhang X, Wang Y, Shen W, Huang L, et al. Ln3+-Induced Diblock Copolymeric Aggregates for Fully Flexible Tunable White-Light Materials. Nanomaterials. 2019; 9(3):363. https://doi.org/10.3390/nano9030363
Chicago/Turabian StyleWang, Xinzhi, Jianguo Tang, Guanghui Wang, Wei Wang, Junjie Ren, Wei Ding, Xinbo Zhang, Yao Wang, Wenfei Shen, Linjun Huang, and et al. 2019. "Ln3+-Induced Diblock Copolymeric Aggregates for Fully Flexible Tunable White-Light Materials" Nanomaterials 9, no. 3: 363. https://doi.org/10.3390/nano9030363





