Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Fabrication of a Single Cd2+-NDS1 NW
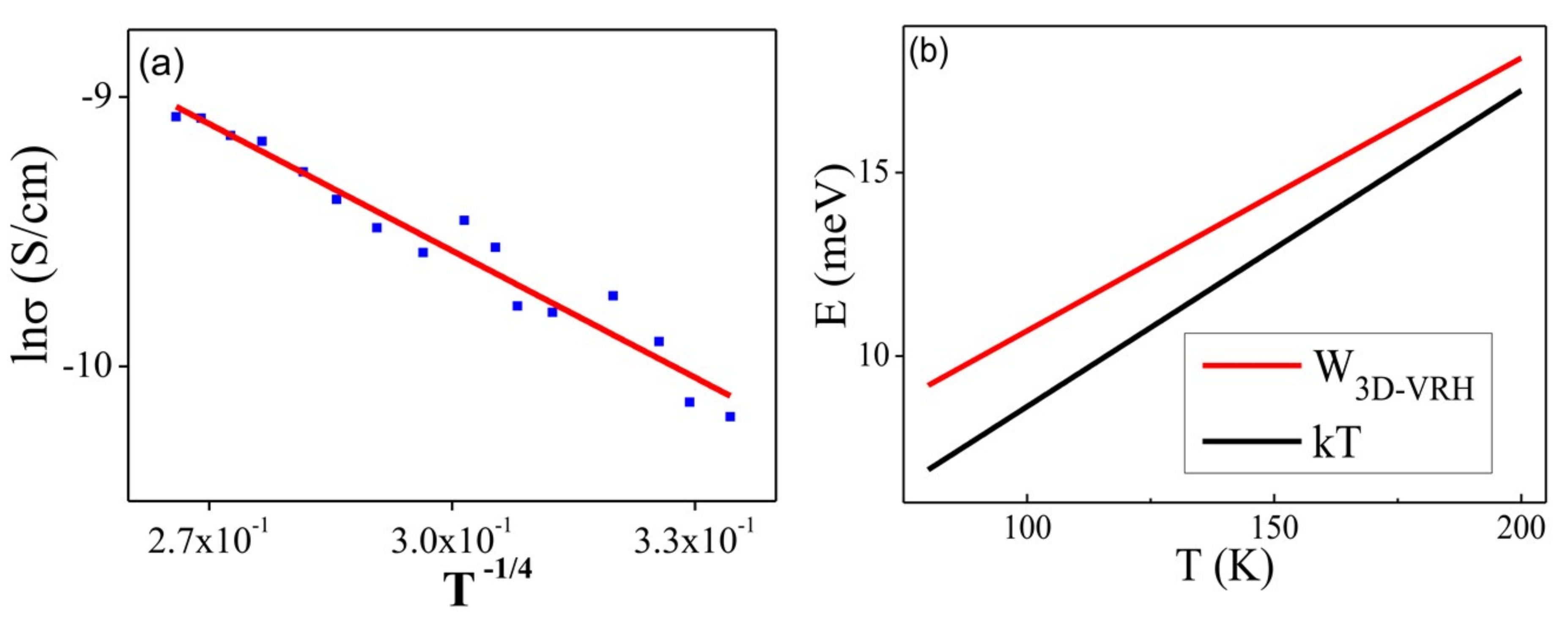
2.3. Activation Energy (Ea) Calculations
2.4. Evaluation of Electron Transport Mechanisms
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Long, Y.-Z.; Yu, M.; Sun, B.; Gu, C.-Z.; Fan, Z. Recent advances in large-scale assembly of semiconducting inorganic nanowires and nanofibers for electronics, sensors and photovoltaics. Chem. Soc. Rev. 2012, 41, 4560–4580. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.P.; Sun, J.; Liu, C.; Brittman, S.; Andrews, S.C.; Lim, J.; Gao, H.; Yan, R.; Yang, P. 25th Anniversary Article: Semiconductor Nanowires—Synthesis, Characterization, and Applications. Adv. Mater. 2014, 26, 2137–2184. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.P.; Pham, T.T.T.; Wolfrum, B.; Offenhäusser, A.; Thierry, B. CMOS-Compatible Silicon Nanowire Field-Effect Transistor Biosensor: Technology Development toward Commercialization. Materials 2018, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Ren, G.; Li, S.; Bi, C.; Hao, Q.; Liu, H. High-Switching-Ratio Photodetectors Based on Perovskite CH3NH3PbI3 Nanowires. Nanomaterials 2018, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Gargas, D.; Yang, P. Nanowire photonics. Nat. Photonics 2009, 3, 569–576. [Google Scholar] [CrossRef]
- Zhong, Z.; Qian, F.; Wang, D.; Lieber, C.M. Synthesis of p-Type Gallium Nitride Nanowires for Electronic and Photonic Nanodevices. Nano Lett. 2003, 3, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Garnett, E.C.; Brongersma, M.L.; Cui, Y.; McGehee, M.D. Nanowire Solar Cells. Annu. Rev. Mater. Res. 2011, 41, 269–295. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor Nanowires for Energy Conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T.; Parthasarathy, R.; Sawicki, G.; Lin, X.-M.; Jaeger, H.; Lindquist, S.L. Conducting nanowires built by controlled self-assembly of amyloid fibers and selective metal deposition. Proc. Nat. Acad. Sci. USA 2003, 100, 4527–4532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.-C.; Wang, S.; Wu, H.; Narasimhan, V.K.; Kong, D.; Lee, H.R.; Cui, Y. Performance enhancement of metal nanowire transparent conducting electrodes by mesoscale metal wires. Nat. Commun. 2013, 4, 2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macias-Montero, M.; Filippin, A.N.; Saghi, Z.; Aparicio, F.J.; Barranco, A.; Espinos, J.P.; Frutos, F.; Gonzalez-Elipe, A.R.; Borras, A. Vertically Aligned Hybrid Core/Shell Semiconductor Nanowires for Photonics Applications. Adv. Funct. Mater. 2013, 23, 5981–5989. [Google Scholar] [CrossRef] [Green Version]
- Briseno, A.L.; Holcombe, T.W.; Boukai, A.I.; Garnett, E.C.; Shelton, S.W.; Fréchet, J.J.M.; Yang, P. Oligo- and Polythiophene/ZnO Hybrid Nanowire Solar Cells. Nano Lett. 2010, 10, 334–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pescaglini, A.; Iacopino, D. Metal nanoparticle–semiconductor nanowire hybrid nanostructures for plasmon-enhanced optoelectronics and sensing. J. Mater. Chem. C 2015, 3, 11785–11800. [Google Scholar] [CrossRef]
- Wang, X.; Sumboja, A.; Lin, M.; Yan, J.; Lee, P.S. Enhancing electrochemical reaction sites in nickel–cobalt layered double hydroxides on zinc tin oxide nanowires: A hybrid material for an asymmetric supercapacitor device. Nanoscale 2012, 4, 7266–7272. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Coffinier, Y.; Boukherroub, R. Diamond Nanowires: A Novel Platform for Electrochemistry and Matrix-Free Mass Spectrometry. Sensors 2015, 15, 12573–12593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Wu, L.; Zhi, J. Diamond Nanowires: Fabrication, Structure, Properties, and Applications. Angew. Chem. Int. Ed. 2014, 53, 14326–14351. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, I.; Lebedev, O.I.; Ralchenko, V.G.; Goovaerts, E.; Bertoni, G.; Van Tendeloo, G.; Konov, V.I. Hybrid Diamond-Graphite Nanowires Produced by Microwave Plasma Chemical Vapor Deposition. Adv. Mater. 2007, 19, 4058–4062. [Google Scholar] [CrossRef]
- Panda, K.; Sankaran, K.J.; Panigrahi, B.K.; Tai, N.-H.; Lin, I.N. Direct Observation and Mechanism for Enhanced Electron Emission in Hydrogen Plasma-Treated Diamond Nanowire Films. ACS Appl. Mater. Interfaces 2014, 6, 8531–8541. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, T.; Demeulemeester, C.; Eyben, P.; Schulz, V.; Richard, O.; Bender, H.; Vandervorst, W. Conductive diamond tips with sub-nanometer electrical resolution for characterization of nanoelectronics device structures. Phys. Status Solidi A 2009, 206, 2077–2081. [Google Scholar] [CrossRef]
- Liao, M.; Hishita, S.; Watanabe, E.; Koizumi, S.; Koide, Y. Suspended Single-Crystal Diamond Nanowires for High-Performance Nanoelectromechanical Switches. Adv. Mater. 2010, 22, 5393–5397. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Sun, K.W. A review on potential applications of diamond nanomaterials. Sci. Adv. Today 2016, 2, 25248. [Google Scholar]
- Arenal, R.; Bruno, P.; Miller, D.; Bleuel, M.; Lal, J.; Gruen, D. Diamond nanowires and the insulator-metal transition in ultrananocrystalline diamond films. Phys. Rev. B 2007, 75, 195431. [Google Scholar] [CrossRef]
- Kholmanov, I.N.; Magnuson, C.W.; Aliev, A.E.; Li, H.; Zhang, B.; Suk, J.W.; Zhang, L.L.; Peng, E.; Mousavi, S.H.; Khanikaev, A.B.; et al. Improved Electrical Conductivity of Graphene Films Integrated with Metal Nanowires. Nano Lett. 2012, 12, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Xu, J. Diamond nanowire—A challenge from extremes. Nanoscale 2012, 4, 5293–5299. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Cloutier, S.G.; Palefsky, S.; Xu, J. Synthesis of Diamond Nanowires Using Atmospheric-Pressure Chemical Vapor Deposition. Nano Lett. 2010, 10, 3272–3276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.; Feng, Y.; Ishiwata, H.; Miyata, Y.; Kitaura, R.; Dahl, J.E.P.; Carlson, R.M.K.; Fokina, N.A.; Schreiner, P.R.; et al. Evidence of Diamond Nanowires Formed inside Carbon Nanotubes from Diamantane Dicarboxylic Acid. Angew. Chem. Int. Ed. 2013, 52, 3717–3721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, J.X.; Chen, X.; Ding, J.W.; Zhang, P.; Ren, W. Diamond nanowires with nitrogen vacancy under a transverse electric field. Phys. Rev. B 2015, 91, 045417. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Ōsawa, E.; Barnard, A.S. Confirmation of the electrostatic self-assembly of nanodiamonds. Nanoscale 2011, 3, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Reusch, M.; Zuerbig, V.; Cimalla, V.; Lee, K.-H.; Kurzyp, M.; Arnault, J.-C.; Nebel, C.; Ambacher, O.; Lebedev, V. Electrostatic Self-Assembly of Diamond Nanoparticles onto Al- and N-Polar Sputtered Aluminum Nitride Surfaces. Nanomaterials 2016, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.; Kriele, A.; Williams, O.A. Electrostatic self-assembly of diamond nanoparticles. Chem. Phys. Lett. 2011, 509, 12–15. [Google Scholar] [CrossRef]
- Jang, D.M.; Im, H.S.; Back, S.H.; Park, K.; Lim, Y.R.; Jung, C.S.; Park, J.; Lee, M. Laser-induced graphitization of colloidal nanodiamonds for excellent oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-S.; Zhao, X. Dangling bond-induced graphitization process on the (111) surface of diamond nanoparticles. J. Chem. Phys. 2011, 134, 044711. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.V.; Gololobov, V.M.; Kononenko, T.V.; Konov, V.I. Photoinduced graphitization of diamond. Laser Phys. Lett. 2015, 12, 016101. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Narayanan, B.; Sankaranarayanan, S.K.R.S.; Yan, Z.; Balandin, A.A.; Zinovev, A.; Rosenmann, D.; Sumant, A.V. Metal-induced rapid transformation of diamond into single and multilayer graphene on wafer scale. Nat. Commun. 2016, 7, 12099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shellaiah, M.; Chen, T.H.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. An Affordable Wet Chemical Route to Grow Conducting Hybrid Graphite-Diamond Nanowires: Demonstration by A Single Nanowire Device. Sci. Rep. 2017, 7, 11243. [Google Scholar] [CrossRef] [PubMed]
- Erdem, E.; Mass, V.; Gembus, A.; Schulz, A.; Liebau-Kunzmann, V.; Fasel, C.; Riedel, R.; Eichel, R.-A. Defect structure in lithium-doped polymer-derived SiCN ceramics characterized by Raman and electron paramagnetic resonance spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 5628–5633. [Google Scholar] [CrossRef] [PubMed]
- Bateni, A.; Erdem, E.; Repp, S.; Acar, S.; Kokal, I.; Häßler, W.; Weber, S.; Somer, M. Electron paramagnetic resonance and Raman spectroscopy studies on carbon-doped MgB2 superconductor nanomaterials. J. Appl. Phys. 2015, 117, 153905. [Google Scholar] [CrossRef]
- García, J.M.; He, R.; Jiang, M.P.; Kim, P.; Pfeiffer, L.N.; Pinczuk, A. Multilayer graphene grown by precipitation upon cooling of nickel on diamond. Carbon 2011, 49, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Cooil, S.P.; Song, F.; Williams, G.T.; Roberts, O.R.; Langstaff, D.P.; Jørgensen, B.; Høydalsvik, K.; Breiby, D.W.; Wahlström, E.; Evans, D.A.; et al. Iron-mediated growth of epitaxial graphene on SiC and diamond. Carbon 2012, 50, 5099–5105. [Google Scholar] [CrossRef]
- Shang, N.; Papakonstantinou, P.; Wang, P.; Zakharov, A.; Palnitkar, U.; Lin, I.N.; Chu, M.; Stamboulis, A. Self-Assembled Growth, Microstructure, and Field-Emission High-Performance of Ultrathin Diamond Nanorods. ACS Nano 2009, 3, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-modified diamond nanoparticles applied in cellular imaging and Hg2+ ions detection. Appl. Surf. Sci. 2019, 465, 340–350. [Google Scholar] [CrossRef]
- Ko, T.-Y.; Shellaiah, M.; Sun, K.W. Thermal and Thermoelectric Transport in Highly Resistive Single Sb2Se3 Nanowires and Nanowire Bundles. Sci. Rep. 2016, 6, 35086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-J.; Jeng, K.-S.; Chen, J.-T.; Sun, K.W. Exceptionally low thermal conductivity of poly(3-hexylthiophene) single nanowires. RSC Adv. 2015, 5, 90847–90851. [Google Scholar] [CrossRef]
- Reddy, K.M.; Padture, N.P.; Punnoose, A.; Hanna, C. Heterojunction metal-oxide-metal Au-Fe3O4-Au single nanowire device for spintronics. J. Appl. Phys. 2015, 117, 17D710. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Lin, Y.-F.; Jian, W.-B.; Chen, H.-C.; Panda, K.; Sundaravel, B.; Dong, C.-L.; Tai, N.-H.; Lin, I.N. Structural and Electrical Properties of Conducting Diamond Nanowires. ACS Appl. Mater. Interfaces 2013, 5, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Nanodiamonds conjugated to gold nanoparticles for colorimetric detection of clenbuterol and chromium(III) in urine. Microchim. Acta 2017, 185, 74. [Google Scholar] [CrossRef] [PubMed]
- Szydlowski, J.; Van Hook, W.A. Concentration and Temperature Dependence of Dynamic Light Scattering for Some Polystyrene Solutions in Toluene, Cyclohexane, Methylcyclohexane and Deuteromethylcyclohexane. Macromolecules 1998, 31, 3266–3274. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Németh, P.; Garvie, L.A.J.; Buseck, P.R. Twinning of cubic diamond explains reported nanodiamond polymorphs. Sci. Rep. 2015, 5, 18381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the Oxygen Content of Graphite and Reduced Graphene Oxide for Specific Applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharisov, B.I.; Kharissova, O.V.; Ortiz Méndez, U. Coordination and organometallic compounds as precursors of classic and less-common nanostructures: Recent advances. J. Coord. Chem. 2013, 66, 3791–3828. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Deng, K.; He, M.; He, L.; Cao, J.; Zhang, X.; Liu, Y.; Li, S.; Tang, Z. Chirality-Discriminated Conductivity of Metal–Amino Acid Biocoordination Polymer Nanowires. ACS Nano 2016, 10, 8564–8570. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jun, Y.-S. The apparent activation energy and pre-exponential kinetic factor for heterogeneous calcium carbonate nucleation on quartz. Commun. Chem. 2018, 1, 56. [Google Scholar] [CrossRef]
- Stallinga, P. Electronic Transport in Organic Materials: Comparison of Band Theory with Percolation/(Variable Range) Hopping Theory. Adv. Mater. 2011, 23, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Ma, J.; Volz, S.; Dumitricǎ, T. Thermally-Active Screw Dislocations in Si Nanowires and Nanotubes. Small 2014, 10, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kato, H.; Oyama, K.; Makino, T.; Ogura, M.; Takeuchi, D.; Inokuma, T.; Tokuda, N.; Yamasaki, S. Inversion channel diamond metal-oxide-semiconductor field-effect transistor with normally off characteristics. Sci. Rep. 2016, 6, 31585. [Google Scholar] [CrossRef] [PubMed]
- Beloborodov, I.S.; Zapol, P.; Gruen, D.M.; Curtiss, L.A. Transport properties of n-type ultrananocrystalline diamond films. Phys. Rev. B 2006, 74, 235434. [Google Scholar] [CrossRef]
- Trucchi, D.M.; Allegrini, P.; Bellucci, A.; Calvani, P.; Galbiati, A.; Girolami, M. Resistant and sensitive single-crystal diamond dosimeters for ionizing radiation. Nucl. Inst. Methods Phys. Res. A 2013, 718, 373–375. [Google Scholar] [CrossRef]
- Lions, M.; Saada, S.; Mazellier, J.-P.; Andrieu, F.; Faynot, O.; Bergonzo, P. Ultra-thin nanocrystalline diamond films (<100 nm) with high electrical resistivity. Phys. Status Solidi RRL 2009, 3, 205–207. [Google Scholar]
- Ma, K.L.; Zhang, W.J.; Zou, Y.S.; Chong, Y.M.; Leung, K.M.; Bello, I.; Lee, S.T. Electrical properties of nitrogen incorporated nanocrystalline diamond films. Diamond Relat. Mater. 2006, 15, 626–630. [Google Scholar] [CrossRef]
- Hsu, C.-H. Realization of New Form of Carbon-Diamond Nanowire–Synthesis, Sharacterization and Applications. Ph.D. Thesis, Brown University, Providence, RI, USA, 2011. [Google Scholar]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Kiuchi, M.; Isono, Y.; Sugiyama, S.; Morita, T.; Matsui, S. Mechanical and electrical properties evaluation of carbon nanowire using electrostatic actuated nano tensile testing devices (EANAT). In Proceedings of the 5th IEEE Conference on Nanotechnology, Nagoya, Japan, 15 July 2005; Volume 2, pp. 486–489. [Google Scholar]


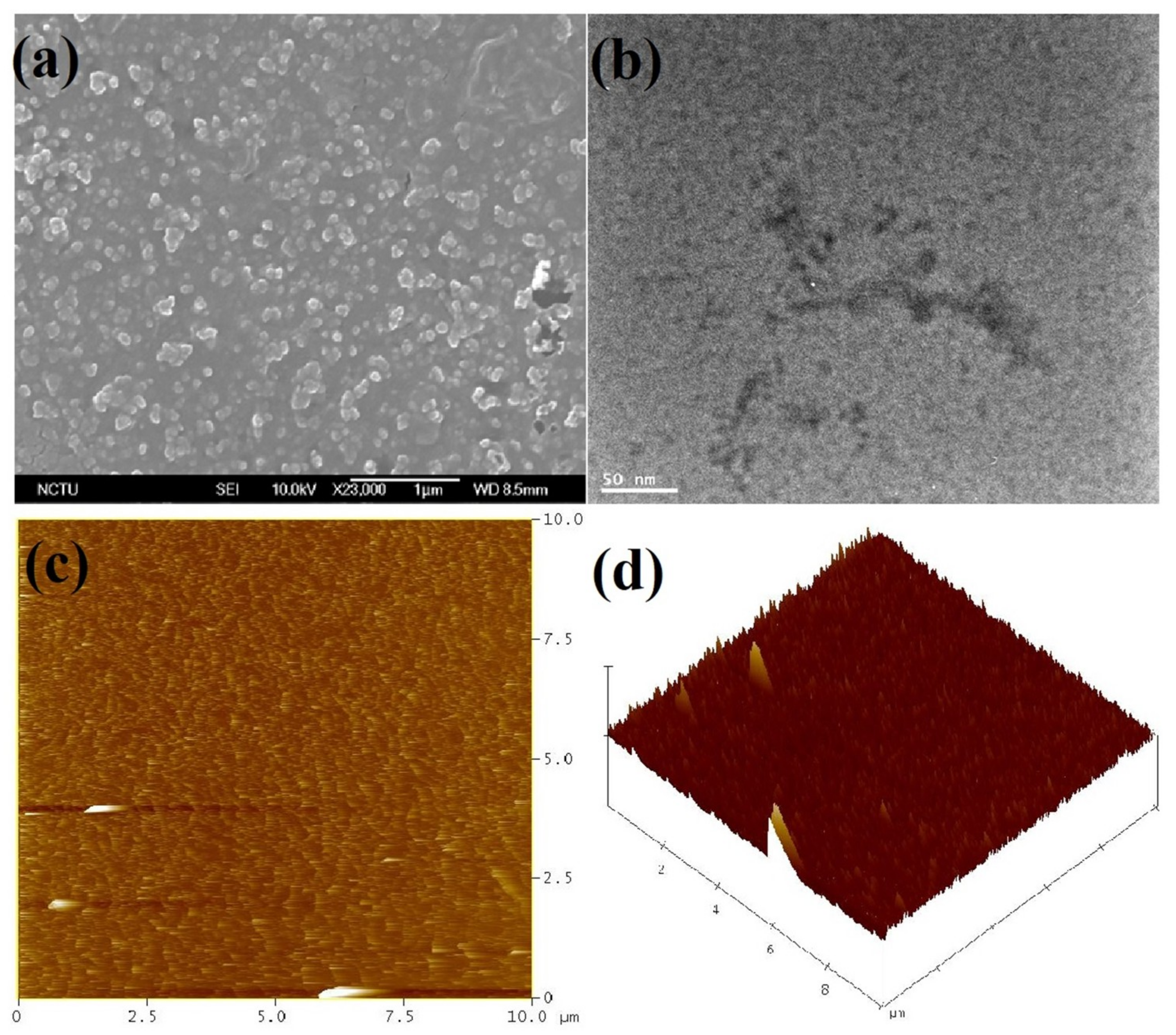








| Compound | C (%) | O (%) | N (%) | S (%) | Cd (%) |
|---|---|---|---|---|---|
| p-ND | 96.21 | 3.79 | - | - | - |
| NDA | 87.32 | 12.68 | - | - | - |
| NDS1 | 59.42 | 17.40 | 17.71 | 5.47 | - |
| Cd2+-NDS1 NWs | 51.60 | 16.51 | 14.46 | 5.22 | 12.21 |
| Device Measurement Condition | Cd2+-NDS1 NW Area | Resistance (G·Ω) | Resistivity (Ω·cm) | Conductivity (mS/cm) | Coefficient of Variation |
|---|---|---|---|---|---|
| Atmosphere (760 torr) | L1 | 7.02 | 3.98 × 104 | 2.51 × 10−5 | 0.66% |
| L2 | 0.78 | 8.70 × 103 | 1.15 × 10−4 | 0.20% | |
| L3 | 4.39 | 4.10 × 104 | 2.44 × 10−5 | 0.51% | |
| Vacuum (10−2 torr) | L1 | 4.55 | 2.58 × 104 | 3.88 × 10−5 | 0.74% |
| L2 | 0.39 | 4.33 × 103 | 2.31 × 10−4 | 0.11% | |
| L3 | 2.91 | 2.72 × 104 | 3.68 × 10−5 | 0.58% |
| Materials | Growth Route/Technique | Electrical Resistivity (Ω-cm) | Ref |
|---|---|---|---|
| Single ND-Cys G-DNW | Wet-Chemical Synthesis | 370.76 | [36] |
| ND-Cys + Hg2+ assembly | Wet-Chemical Synthesis | NA | [42] |
| Undoped-UNCD | NA | 106 | [58] |
| Single Crystal Diamond | Commercial Source | 1014 | [59] |
| Ultra-thin Nanocrystalline Diamond Films | MPCVD | 5 × 1013 | [60] |
| Nitrogen Incorporated Diamond Films | MPCVD | 105 | [61] |
| Single Diamond Nanowire | APCVD | NA | [62] |
| Carbon Nanotube | Carbon-arc Method | 10−3~10−6 | [63] |
| Carbon Nanowire | NA | 0.015 | [64] |
| Single Cd2+-NDS1 NW | Wet-Chemical Synthesis | 4.33 × 103 | This Work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Chen, Y.-C.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device. Nanomaterials 2019, 9, 415. https://doi.org/10.3390/nano9030415
Shellaiah M, Chen Y-C, Simon T, Li L-C, Sun KW, Ko F-H. Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device. Nanomaterials. 2019; 9(3):415. https://doi.org/10.3390/nano9030415
Chicago/Turabian StyleShellaiah, Muthaiah, Ying-Chou Chen, Turibius Simon, Liang-Chen Li, Kien Wen Sun, and Fu-Hsiang Ko. 2019. "Effect of Metal Ions on Hybrid Graphite-Diamond Nanowire Growth: Conductivity Measurements from a Single Nanowire Device" Nanomaterials 9, no. 3: 415. https://doi.org/10.3390/nano9030415







