Near-Infrared Surface-Enhanced Raman Scattering on Silver-Coated Porous Silicon Photonic Crystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Silicon Photoluminescence
3.2. Porous Silicon Photonic Crystals
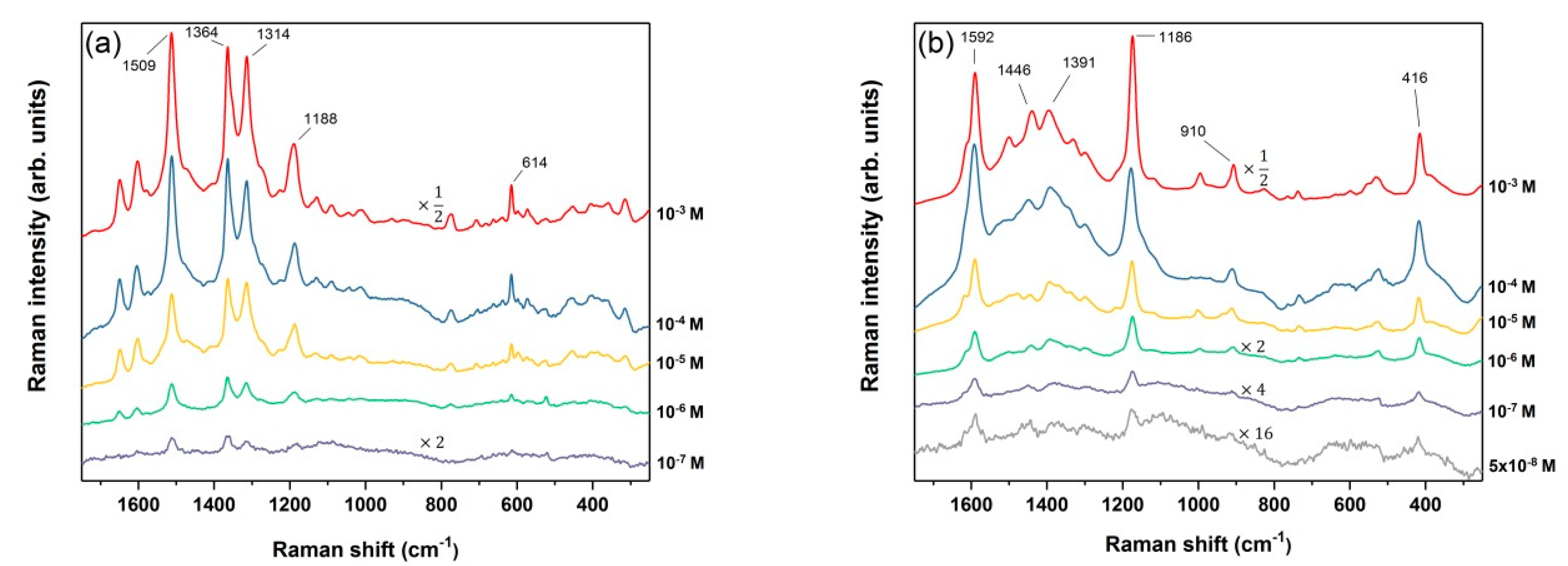
3.3. SERS on Porous Silicon Photonic Crystals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moskovits, M. Surface-Enhanced Raman Spectroscopy: A Brief Retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, S.; Zang, L.; Lu, X.; Zhang, H. Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, P.G.; Le Ru, E.C. A Perspective on Single Molecule SERS: Current Status and Future Challenges. Phys. Chem. Chem. Phys. 2008, 10, 6079–6089. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, J.R.; Birke, R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosier-Boss, P. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]
- Chan, S.; Kwon, S.; Koo, T.W.; Lee, L.P.; Berlin, A.A. Surface-Enhanced Raman Scattering of Small Molecules from Silver-Coated Silicon Nanopores. Adv. Mater. 2003, 15, 1595–1598. [Google Scholar] [CrossRef]
- Lin, H.; Mock, J.; Smith, D.; Gao, T.; Sailor, M.J. Surface-Enhanced Raman Scattering from Silver-Plated Porous Silicon. J. Phys. Chem. B 2004, 108, 11654–11659. [Google Scholar] [CrossRef]
- Giorgis, F.; Descrovi, E.; Chiodoni, A.; Froner, E.; Scarpa, M.; Venturello, A.; Geobaldo, F. Porous Silicon as Efficient Surface Enhanced Raman Scattering (SERS) Substrate. Appl. Surf. Sci. 2008, 254, 7494–7497. [Google Scholar] [CrossRef]
- Panarin, A.Y.; Terekhov, S.N.; Kholostov, K.I.; Bondarenko, V.P. SERS-Active Substrates Based on n-Type Porous Silicon. Appl. Surf. Sci. 2010, 256, 6969–6976. [Google Scholar] [CrossRef]
- Zeiri, L.; Rechav, K.; Porat, Z.; Zeiri, Y. Silver Nanoparticles Deposited on Porous Silicon as a Surface- Enhanced Raman Scattering (SERS) Active Substrate. Appl. Spectrosc. 2012, 66, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A.; Ismail, A.A.; Bouzid, H.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Surface-Enhanced Raman Scattering (SERS)-Active Substrates from Silver Plated-Porous Silicon for Detection of Crystal Violet. Appl. Surf. Sci. 2015, 331, 241–247. [Google Scholar] [CrossRef]
- Kosovic, M.; Balarin, M.; Ivanda, M.; Derek, V.; Marcius, M.; Ristic, M.; Gamulin, O. Porous Silicon Covered with Silver Nanoparticles as Surface-Enhanced Raman Scattering (SERS) Substrate for Ultra-Low Concentration Detection. Appl. Spectrosc. 2015, 69, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Bandarenka, H.V.; Girel, K.V.; Zavatski, S.A.; Panarin, A.; Terekhov, S.N. Progress in the Development of SERS-Active Substrates Based on Metal-Coated Porous Silicon. Materials 2018, 11, 852. [Google Scholar] [CrossRef]
- Canham, L. Handbook of Porous Silicon; Springer International Publishing: Berlin, Germany, 2014. [Google Scholar]
- Sailor, M.J. Porous Silicon in Practice: Preparation, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Andsager, D.; Hilliard, J.; Hetrick, J.M.; Abuhassan, L.H.; Plisch, M.; Nayfeh, M.H. Quenching of Porous Silicon Photoluminescence by Deposition of Metal Adsorbates. J. Appl. Phys. 1993, 74, 4783–4785. [Google Scholar] [CrossRef]
- Harraz, F.A.; Tsuboi, T.; Sasano, J.; Sakka, T.; Ogata, Y.H. Metal Deposition onto a Porous Silicon Layer by Immersion Plating from Aqueous and Nonaqueous Solutions. J. Electrochem. Soc. 2002, 149, C456–C463. [Google Scholar] [CrossRef]
- Nativ-Roth, E.; Rechav, K.; Porat, Z. Deposition of Gold and Silver on Porous Silicon and Inside the Pores. Thin Solid Films 2016, 603, 88–96. [Google Scholar] [CrossRef]
- Chursanova, M.V.; Germash, L.P.; Yukhymchuk, V.O.; Dzhagan, V.M.; Khodasevich, I.A.; Cojoc, D. Optimization of Porous Silicon Preparation Technology for SERS Applications. Appl. Surf. Sci. 2010, 256, 3369–3373. [Google Scholar] [CrossRef]
- Bandarenka, H.; Redko, S.; Nenzi, P.; Balucani, M.; Bondarenko, V. Optimization of Chemical Displacement Deposition of Copper on Porous Silicon. J. Nanosci. Nanotechnol. 2012, 12, 8725–8731. [Google Scholar] [CrossRef]
- Mikac, L.; Ivanda, M.; Đerek, V.; Gotić, M. Influence of Mesoporous Silicon Preparation Condition on Silver Clustering and SERS Enhancement. J. Raman Spectrosc. 2016, 47, 1036–1041. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer Science+Business Media LLC: New York, NY, USA, 2007. [Google Scholar]
- Kneipp, K.; Roth, E.; Engert, C.; Kiefer, W. Near-Infrared Excited Surface-Enhanced Raman Spectroscopy of Rhodamine 6G on Colloidal Silver. Chem. Phys. Lett. 1993, 207, 450–454. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Raman and Computational Study on the Adsorption of Xanthine on Silver Nanocolloids. ACS Omega 2018, 3, 13530–13537. [Google Scholar] [CrossRef]
- DaFonseca, B.G.; Costa, L.A.S.; Sant’Ana, A.C. Insights of Adsorption Mechanisms of Trp-Peptides on Plasmonic Surfaces by SERS. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 190, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.M.; Katz, L.F.; Archibald, D.D.; Lin, L.T.; Honigs, D.E. Near-Infrared Surface-Enhanced Raman Spectroscopy. Part 1: Copper and Gold Electrodes. Appl. Spectrosc. 1988, 42, 1327–1331. [Google Scholar] [CrossRef]
- Hildebrandt, P.; Keller, S.; Hoffmann, A.; Vanhecke, F.; Schrader, B. Enhancement Factor of Surface-Enhanced Raman Scattering on Silver and Gold Surfaces Upon Near-Infrared Excitation. Indication of an Unusual Strong Contribution of the Chemical Effect. J. Raman Spectrosc. 1993, 24, 791–796. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Surface-Enhanced Raman Scattering Studies on Aggregated Gold Nanorods. J. Phys. Chem. A 2003, 107, 3372–3378. [Google Scholar] [CrossRef]
- Lai, C.H.; Wang, G.A.; Ling, T.K.; Wang, T.J.; Chiu, P.K.; Chou Chau, Y.F.; Huang, C.C.; Chiang, H.P. Near Infrared Surface-Enhanced Raman Scattering Based on Star-Shaped Gold/Silver Nanoparticles and Hyperbolic Metamaterial. Sci. Rep. 2017, 7, 5446. [Google Scholar] [CrossRef]
- Dash, W.C.; Newman, R. Intrinsic Optical Absorption in Single-Crystal Germanium and Silicon at 77°K and 300°K. Phys. Rev. 1955, 99, 1151–1155. [Google Scholar] [CrossRef]
- Sagnes, I.; Halimaoui, A.; Vincent, G.; Badoz, P.A. Optical Absorption Evidence of a Quantum Size Effect in Porous Silicon. Appl. Phys. Lett. 1993, 62, 1155–1157. [Google Scholar] [CrossRef]
- Webb, J.D.; Dunlavy, D.J.; Ciszek, T.; Ahrenkiel, R.K.; Wanlass, M.W.; Noufi, R.; Vernon, S.M. Room-Temperature Measurement of Photoluminescence Spectra of Semiconductors Using an FT-Raman Spectrophotometer. Appl. Spectrosc. 1993, 47, 1814–1819. [Google Scholar] [CrossRef]
- Kosović, M.; Gamulin, O.; Balarin, M.; Ivanda, M.; Đerek, V.; Ristić, D.; Marciuš, M.; Ristić, M. Phonon Confinement Effects in Raman Spectra of Porous Silicon at Non-Resonant Excitation Condition. J. Raman Spectrosc. 2014, 45, 470–475. [Google Scholar] [CrossRef]
- Mazzoleni, C.; Pavesi, L. Application to Optical Components of Dielectric Porous Silicon Multilayers. Appl. Phys. Lett. 1995, 67, 2983. [Google Scholar] [CrossRef]
- Berger, M.G.; Arens-Fischer, R.; Thönissen, M.; Krüger, M.; Billat, S.; Lüth, H.; Hilbrich, S.; Theiß, W.; Grosse, P. Dielectric Filters Made of PS: Advanced Performance by Oxidation and New Layer Structures. Thin Solid Films 1997, 297, 237–240. [Google Scholar] [CrossRef]
- Agarwal, V.; Del Río, J.A. Tailoring the Photonic Band Gap of a Porous Silicon Dielectric Mirror. Appl. Phys. Lett. 2003, 82, 1512–1514. [Google Scholar] [CrossRef]
- Pacholski, C. Photonic Crystal Sensors Based on Porous Silicon. Sensors 2013, 13, 4694–4713. [Google Scholar] [CrossRef] [Green Version]
- Harraz, F.A. Porous Silicon Chemical Sensors and Biosensors: A Review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Ferrand, P.; Romestain, R.; Vial, J.C. Photonic Band-Gap Properties of a Porous Silicon Periodic Planar Waveguide. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 115106. [Google Scholar] [CrossRef]
- Cunin, F.; Schmedake, T.A.; Link, J.R.; Li, Y.Y.; Kom, J.; Bhatia, S.N.; Sailor, M.J. Biomolecular Screening with Encoded Porous-Silicon Photonic Crystals. Nat. Mater. 2002, 1, 39–41. [Google Scholar] [CrossRef]
- Pavesi, L.; Cazzanelli, M. Porous Silicon Multilayers and Microcavities. Riv. Nuovo Cim. 1997, 20, 1–76. [Google Scholar] [CrossRef]
- Mikac, L.; Ivanda, M.; Gotić, M.; Maksimović, A.; Trusso, S.; Andrea, C.D.; Foti, A.; Irrera, A.; Fazio, B.; Gucciardi, P.G. Metal Nanoparticles Deposited on Porous Silicon Templates as Novel Substrates for SERS. Croat. Chem. Acta 2015, 88, 437–444. [Google Scholar] [CrossRef]
- Haynes, J.R.; Westphal, W.C. Radiation Resulting from Recombination of Holes and Electrons in Silicon. Phys. Rev. 1956, 101, 1676–1678. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Cornet, A.; Morante, J.R.; Jiménez, J.; Hemment, P.L.F.; Homewood, K.P. Raman Scattering and Photoluminescence Analysis of Silicon on Insulator Structures Obtained by Single and Multiple Oxygen Implants. J. Appl. Phys. 1991, 70, 1678–1683. [Google Scholar] [CrossRef]
- Saito, Y.; Wang, J.J.; Smith, D.A.; Batchelder, D.N. A Simple Chemical Method for the Preparation of Silver Surfaces for Efficient SERS. Langmuir 2002, 18, 2000–2002. [Google Scholar] [CrossRef]
- Saito, Y.; Wang, J.J.; Batchelder, D.N.; Smith, D.A. Simple Chemical Method for Forming Silver Surfaces with Controlled Grain Sizes for Surface Plasmon Experiments. Langmuir 2003, 19, 6857–6861. [Google Scholar] [CrossRef]
- Jeske, M.; Schultze, J.W.; Thonissen, M.; Munder, H. Electrodeposition of Metals Into Porous Silicon. Thin Solid Films 1995, 255, 63–66. [Google Scholar] [CrossRef]
- Fox, M. Optical Properties of Solids, 2nd ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Lorenzo, E.; Oton, C.J.; Capuj, N.E.; Ghulinyan, M.; Navarro-Urrios, D.; Gaburro, Z.; Pavesi, L. Porous Silicon-Based Rugate Filters. Appl. Opt. 2005, 44, 5415. [Google Scholar] [CrossRef]
- Li, S.; Hu, D.; Huang, J.; Cai, L. Optical Sensing Nanostructures for Porous Silicon Rugate Filters. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.; Sailor, M.J.; Sakka, T.; Ogata, Y.H. Electrochemical Preparation of a Rugate Filter in Silicon and its Deviation from the Ideal Structure. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Imenes, A.G.; McKenzie, D.R. Flat-Topped Broadband Rugate Filters. Appl. Opt. 2006, 45, 7841–7850. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Böcking, T.; Kilian, K.; Reece, P.J.; Gooding, J.; Gaus, K.; Gal, M. Porous Silicon Based Narrow Line-Width Rugate Filters. Opt. Mater. 2007, 29, 619–622. [Google Scholar] [CrossRef]
- Thönissen, M.; Berger, M.G.; Billat, S.; Arens-Fischer, R.; Krüger, M.; Lüth, H.; Theiß, W.; Hillbrich, S.; Grosse, P.; Lerondel, G.; et al. Analysis of the Depth Homogeneity of p-PS by Reflectance Measurements. Thin Solid Films 1997, 297, 92–96. [Google Scholar] [CrossRef]
- Urata, T.; Fukami, K.; Takeda, N.; Sakka, T.; Ogata, Y.H. Influence of Solvents in HF Solutions on Anodic Formation of Mesoporous Silicon, Revealed by the Characterization of Mesoporous Silicon Rugate Filters. ECS J. Solid State Sci. Technol. 2016, 5, P250–P255. [Google Scholar] [CrossRef]
- Michaels, A.M.; Jiang, J.; Brus, L. Ag Nanocrystal Junctions as the Site for Surface-Enhanced Raman Scattering of Single Rhodamine 6G Molecules. J. Phys. Chem. A 2000, 104, 11965–11971. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, P.; Stockburger, M. Surface-Enhanced Resonance Raman Spectroscopy of Rhodamine 6G Adsorbed on Colloidal Silver. J. Phys. Chem. 1984, 88, 5935–5944. [Google Scholar] [CrossRef]
- Majoube, M.; Henry, M. Fourier Transform Raman and Infrared and Surface-Enhanced Raman Spectra for Rhodamine 6G. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1991, 47, 1459–1466. [Google Scholar] [CrossRef]
- Liang, E.J.; Ye, X.L.; Kiefer, W. Surface-Enhanced Raman Spectroscopy of Crystal Violet in the Presence of Halide and Halate Ions with Near-Infrared Wavelength Excitation. J. Phys. Chem. 1997, 101, 7330–7335. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Quantifying SERS Enhancements. MRS Bull. 2013, 38, 631–640. [Google Scholar] [CrossRef]
- Virga, A.; Rivolo, P.; Descrovi, E.; Chiolerio, A.; Digregorio, G.; Frascella, F.; Soster, M.; Bussolino, F.; Marchiò, S.; Geobaldo, F.; et al. SERS Active Ag Nanoparticles in Mesoporous Silicon: Detection of Organic Molecules and Peptide—Antibody Assays. J. Raman Spectrosc. 2011, 43, 730–736. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles the Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Starowicz, Z.; Ozga, P.; Sheregii, E.M. The Tuning of the Plasmon Resonance of the Metal Nanoparticles in Terms of the SERS Effect. Colloid Polym. Sci. 2018, 296, 1029–1037. [Google Scholar] [CrossRef]
- Liao, P.F.; Wokaun, A. Lightning Rod Effect in Surface Enhanced Raman Scattering. J. Chem. Phys. 1982, 76, 751–752. [Google Scholar] [CrossRef]
- Kudelski, A. Raman Studies of Rhodamine 6G and Crystal Violet Sub-Monolayers on Electrochemically Roughened Silver Substrates: Do Dye Molecules Adsorb Preferentially on Highly SERS-Active Sites? Chem. Phys. Lett. 2005, 414, 271–275. [Google Scholar] [CrossRef]
- Natan, M.J. Concluding Remarks - Surface Enhanced Raman Scattering. Faraday Discuss. 2006, 132, 321–328. [Google Scholar] [CrossRef]
- Wu, C.Y.; Huang, C.C.; Jhang, J.S.; Liu, A.C.; Chiang, C.; Hsieh, M.-L.; Huang, P.-J.; Tuyen, L.D.; Minh, L.Q.; Yang, S.T.; et al. Hybrid Surface-Enhanced Raman Scattering Substrate from Gold Nanoparticle and Photonic Crystal: Maneuverability and Uniformity of Raman Spectra. Opt. Express 2009, 17, 21522–21529. [Google Scholar] [CrossRef]
- Mamichev, D.A.; Gonchar, K.A.; Timoshenko, V.Y.; Mussabek, G.K.; Nikulin, V.E.; Taurbaev, T.I. Enhanced Raman Scattering in Multilayer Structures of Porous Silicon. J. Raman Spectrosc. 2011, 42, 1392–1395. [Google Scholar] [CrossRef]
- Tuyen, L.D.; Liu, A.C.; Huang, C.; Tsai, P.; Lin, J.H.; Wu, C.; Chau, L.; Yang, T.S.; Minh, L.Q.; Kan, H.; et al. Doubly Resonant Surface-Enhanced Raman Scattering on Gold Nanorod Decorated Inverse Opal Photonic Crystals. Opt. Express 2012, 20, 29266–29275. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Wu, Z.; Guo, J.; Jia, D. Porous Silicon Photonic Crystals Coated with Ag Nanoparticles as Efficient Substrates for Detecting Trace Explosives Using SERS. Nanomaterials 2018, 8, 872. [Google Scholar] [CrossRef]
- Ishikura, N.; Fujii, M.; Nishida, K.; Hayashi, S.; Diener, J.; Mizuhata, M.; Deki, S. Broadband Rugate Filters Based on Porous Silicon. Opt. Mater. 2008, 31, 102–105. [Google Scholar] [CrossRef]




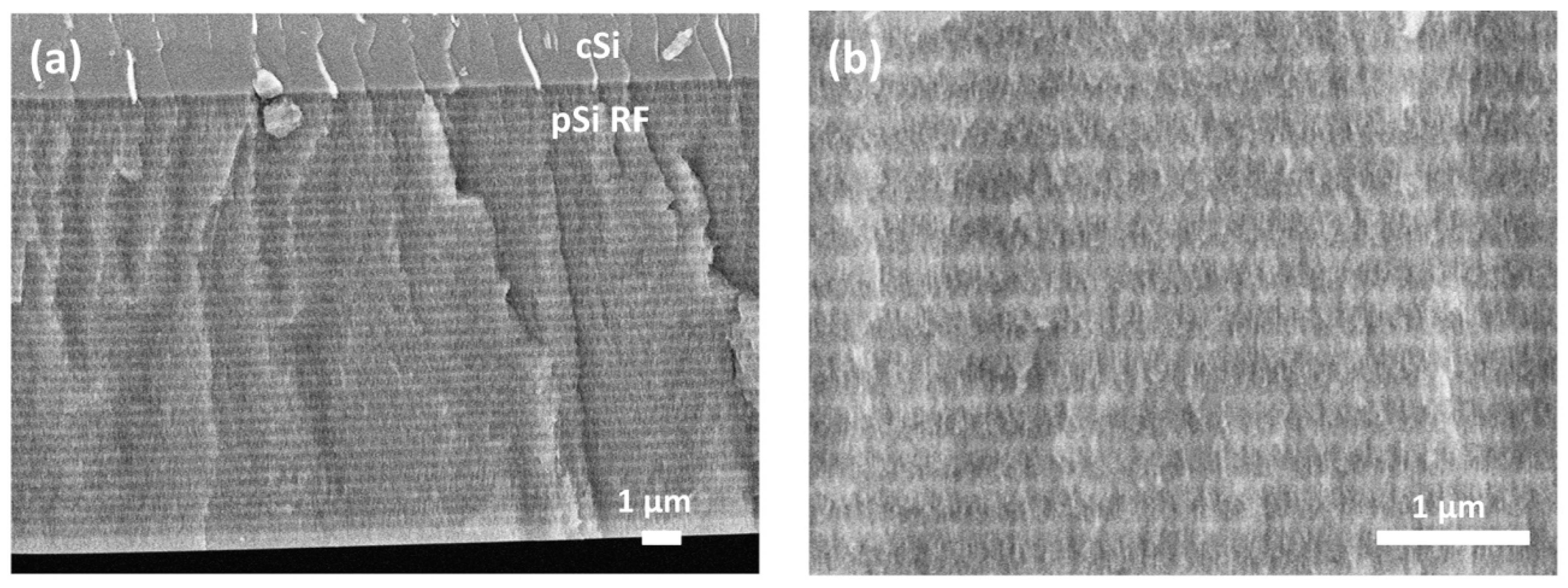



| Sample | J (mA/cm2) | Period (s) | Cycles |
|---|---|---|---|
| pSi S1 | 50.5 | n/a | n/a |
| RF S1 | 1–100 | 4.6 | 40 |
| RF S2 | 1–100 | 4.7 | 40 |
| RF S2a | 1–100 | 4.7 | 80 |
| RF S3 | 1–100 | 4.8 | 40 |
| RF S4 | 1–100 | 4.9 | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrabić, M.; Kosović, M.; Gotić, M.; Mikac, L.; Ivanda, M.; Gamulin, O. Near-Infrared Surface-Enhanced Raman Scattering on Silver-Coated Porous Silicon Photonic Crystals. Nanomaterials 2019, 9, 421. https://doi.org/10.3390/nano9030421
Škrabić M, Kosović M, Gotić M, Mikac L, Ivanda M, Gamulin O. Near-Infrared Surface-Enhanced Raman Scattering on Silver-Coated Porous Silicon Photonic Crystals. Nanomaterials. 2019; 9(3):421. https://doi.org/10.3390/nano9030421
Chicago/Turabian StyleŠkrabić, Marko, Marin Kosović, Marijan Gotić, Lara Mikac, Mile Ivanda, and Ozren Gamulin. 2019. "Near-Infrared Surface-Enhanced Raman Scattering on Silver-Coated Porous Silicon Photonic Crystals" Nanomaterials 9, no. 3: 421. https://doi.org/10.3390/nano9030421






