Characterization of Vertically Aligned Carbon Nanotube Forests Grown on Stainless Steel Surfaces
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Investigation
3.2. Quasi-Static Characterization
3.3. Wetting Properties
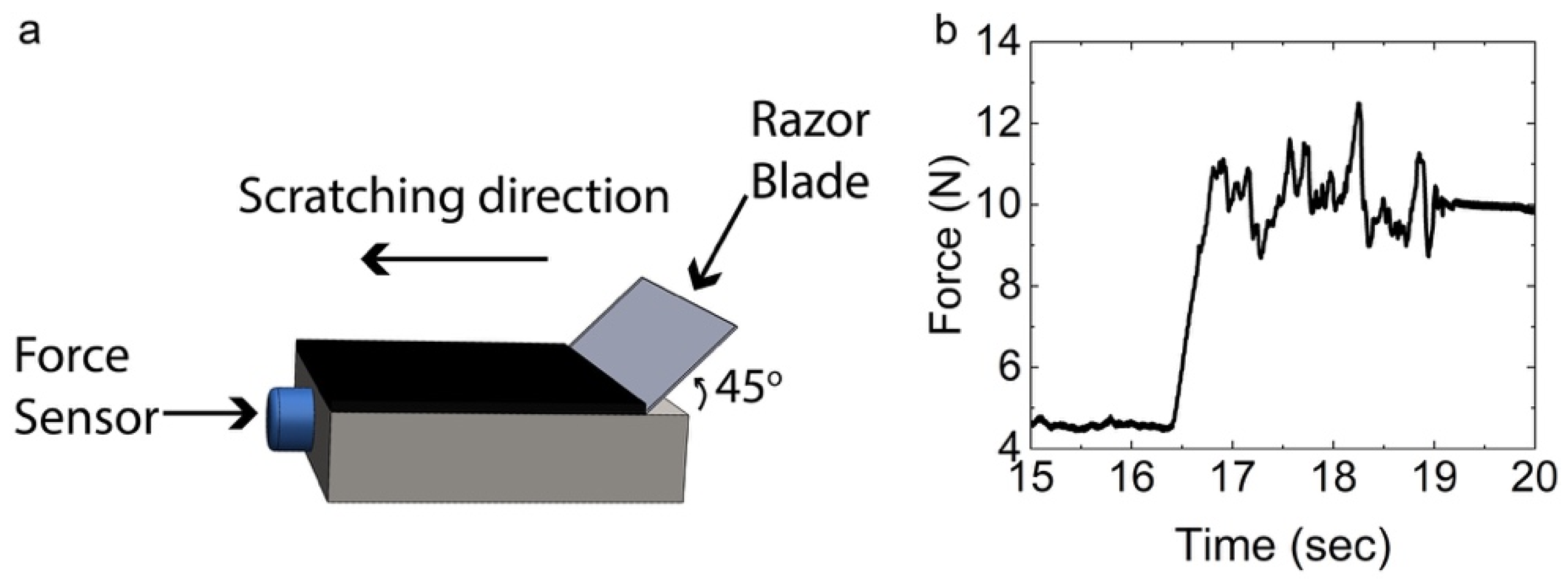
3.4. Adhesion Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, A.; Dickrell, P.L.; Sawyer, W.G.; Ghasemi-Nejhad, M.N.; Ajayan, P.M. Super-Compressible Foamlike Carbon Nanotube Films. Science 2005, 310, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dai, L.; Stone, M.; Xia, Z.; Wang, Z.L. Carbon Nanotube Arrays with Strong Shear Binding-On and Easy Normal Lifting-Off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2014, 535, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef]
- Thevamaran, R.; Meshot, E.R.; Daraio, C. Shock Formation and Rate Effects in Impacted Carbon Nanotube Foams. Carbon 2015, 84, 390–398. [Google Scholar] [CrossRef]
- Raney, J.R.; Misra, A.; Daraio, C. Tailoring the Microstructure and Mechanical Properties of Arrays of Aligned Multiwall Carbon Nanotubes by Utilizing Different Hydrogen Concentrations during Synthesis. Carbon 2011, 49, 3631–3638. [Google Scholar] [CrossRef]
- Thevamaran, R.; Karakaya, M.; Meshot, E.R.; Fischer, A.; Podila, R.; Rao, A.M.; Daraio, C. Anomalous Impact and Strain Responses in Helical Carbon Nanotube Foams. RSC Adv. 2015, 5, 29306–29311. [Google Scholar] [CrossRef]
- Pour Shahid Saeed Abadi, P.; Hutchens, S.B.; Greer, J.R.; Cola, B.A.; Graham, S. Effects of Morphology on the Micro-Compression Response of Carbon Nanotube Forests. Nanoscale 2012, 4, 3373. [Google Scholar] [CrossRef] [PubMed]
- Yaglioglu, O.; Cao, A.; Hart, A.J.; Martens, R.; Slocum, A.H. Wide Range Control of Microstructure and Mechanical Properties of Carbon Nanotube Forests: A Comparison between Fixed and Floating Catalyst CVD Techniques. Adv. Funct. Mater. 2012, 22, 5028–5037. [Google Scholar] [CrossRef]
- Pathak, S.; Raney, J.R.; Daraio, C. Effect of Morphology on the Strain Recovery of Vertically Aligned Carbon Nanotube Arrays: An in Situ Study. Carbon 2013, 63, 303–316. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Hall, L.J. Carbon Nanotube Synthesis upon Stainless Steel Meshes. Carbon 2003, 41, 659–672. [Google Scholar] [CrossRef]
- Talapatra, S.; Kar, S.; Pal, S.K.; Vajtai, R.; Ci, L.; Victor, P.; Shaijumon, M.M.; Kaur, S.; Nalamasu, O.; Ajayan, P.M. Direct Growth of Aligned Carbon Nanotubes on Bulk Metals. Nat. Nano 2006, 1, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Karwa, M.; Iqbal, Z.; Mitra, S. Scaled-up Self-Assembly of Carbon Nanotubes inside Long Stainless Steel Tubing. Carbon 2006, 44, 1235–1242. [Google Scholar] [CrossRef]
- Cao, A.; Veedu, V.P.; Xuesong, L.I.; Yao, Z.; Ghasemi-Nejhad, M.N.; Ajayan, P.M. Multifunctional Brushes Made from Carbon Nanotubes. Nat. Mater. 2005, 4, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Masarapu, C.; Wei, B. Direct Growth of Aligned Multiwalled Carbon Nanotubes on Treated Stainless Steel Substrates. Langmuir 2007, 23, 9046–9049. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Hori, Y.; Yamamoto, S.; Tamon, H. A Simple Oxidation-Reduction Process for the Activation of a Stainless Steel Surface to Synthesize Multi-Walled Carbon Nanotubes and Its Application to Phenol Degradation in Water. Carbon 2012, 50, 115–122. [Google Scholar] [CrossRef]
- Hordy, N.; Mendoza-Gonzalez, N.Y.; Coulombe, S.; Meunier, J.L. The Effect of Carbon Input on the Morphology and Attachment of Carbon Nanotubes Grown Directly from Stainless Steel. Carbon 2013, 63, 348–357. [Google Scholar] [CrossRef]
- Sano, N.; Yamamoto, S.; Tamon, H. Cr as a Key Factor for Direct Synthesis of Multi-Walled Carbon Nanotubes on Industrial Alloys. Chem. Eng. J. 2014, 242, 278–284. [Google Scholar] [CrossRef]
- Zhuo, C.; Wang, X.; Nowak, W.; Levendis, Y.A. Oxidative Heat Treatment of 316L Stainless Steel for Effective Catalytic Growth of Carbon Nanotubes. Appl. Surf. Sci. 2014, 313, 227–236. [Google Scholar] [CrossRef]
- Romero, P.; Oro, R.; Campos, M.; Torralba, J.M.; De Villoria, R.G. Simultaneous Synthesis of Vertically Aligned Carbon Nanotubes and Amorphous Carbon Thin Films on Stainless Steel. Carbon 2015, 82, 31–38. [Google Scholar] [CrossRef]
- Reinhardt, H.; Hellmann, C.; Nürnberger, P.; Kachel, S.; Hampp, N. Free Form Growth of Carbon Nanotube Microarchitectures on Stainless Steel Controlled via Laser-Stimulated Catalyst Formation. Adv. Mater. Interfaces 2017, 4, 1700508. [Google Scholar] [CrossRef]
- Camilli, L.; Scarselli, M.; Del Gobbo, S.; Castrucci, P.; Nanni, F.; Gautron, E.; Lefrant, S.; De Crescenzi, M. The Synthesis and Characterization of Carbon Nanotubes Grown by Chemical Vapor Deposition Using a Stainless Steel Catalyst. Carbon 2011, 49, 3307–3315. [Google Scholar] [CrossRef]
- Hashempour, M.; Vicenzo, A.; Zhao, F.; Bestetti, M. Direct Growth of MWCNTs on 316 Stainless Steel by Chemical Vapor Deposition: Effect of Surface Nano-Features on CNT Growth and Structure. Carbon 2013, 63, 330–347. [Google Scholar] [CrossRef]
- Pattinson, S.W.; Viswanath, B.; Zakharov, D.N.; Li, J.; Stach, E.A.; Hart, A.J. Mechanism and Enhanced Yield of Carbon Nanotube Growth on Stainless Steel by Oxygen-Induced Surface Reconstruction. Chem. Mater. 2015, 27, 932–937. [Google Scholar] [CrossRef]
- Pakdee, U.; Chiangga, S.; Suwannatus, S.; Limsuwan, P. Growth of MWCNTs on Flexible Stainless Steels without Additional Catalysts. J. Nanomater. 2017, 2017, 5672728. [Google Scholar] [CrossRef]
- Raja Noor Amalina Raja, S.; Mohd Asyadi, A.; Mohd Ambri, M.; Seman, R.N.A.R.; Azam, M.A.; Mohamed, M.A. Highly Efficient Growth of Vertically Aligned Carbon Nanotubes on Fe–Ni Based Metal Alloy Foils for Supercapacitors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 45016. [Google Scholar] [CrossRef]
- Li, D.; Cheng, Y.; Wang, Y.; Zhang, H.; Dong, C.; Li, D. Improved Field Emission Properties of Carbon Nanotubes Grown on Stainless Steel Substrate and Its Application in Ionization Gauge. Appl. Surf. Sci. 2016, 365, 10–18. [Google Scholar] [CrossRef]
- Park, D.; Kim, Y.H.; Lee, J.K. Synthesis of Carbon Nanotubes on Metallic Substrates by a Sequential Combination of PECVD and Thermal CVD. Carbon 2003, 41, 1025–1029. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, D.G. Development of CNT-Metal-Filters by Direct Growth of Carbon Nanotubes. Curr. Appl. Phys. 2006, 6, 182–186. [Google Scholar] [CrossRef]
- Camilli, L.; Scarselli, M.; Del Gobbo, S.; Castrucci, P.; Lamastra, F.R.; Nanni, F.; Gautron, E.; Lefrant, S.; D’Orazio, F.; Lucari, F.; et al. High Coercivity of Iron-Filled Carbon Nanotubes Synthesized on Austenitic Stainless Steel. Carbon 2012, 50, 718–721. [Google Scholar] [CrossRef]
- Kruehong, S.; Kruehong, C.; Artnaseaw, A. Branched Carbon Fibres and Other Carbon Nanomaterials Grown Directly from 304 Stainless Steel Using a Chemical Vapour Deposition Process. Diam. Relat. Mater. 2016, 64, 143–152. [Google Scholar] [CrossRef]
- Weiwei, Z.; Yu, Z.; Ningsheng, X.; Yuanming, T.; Runze, Z.; Yan, S.; Zhi, X.; Xuedong, B.; Jun, C.; Juncong, S.; et al. Epitaxial Growth of Multiwall Carbon Nanotube from Stainless Steel Substrate and Effect on Electrical Conduction and Field Emission. Nanotechnology 2017, 28, 305704. [Google Scholar]
- Ashraf, A.; Salih, H.; Nam, S.; Dastgheib, S.A. Robust Carbon Nanotube Membranes Directly Grown on Hastelloy Substrates and Their Potential Application for Membrane Distillation. Carbon 2016, 106, 243–251. [Google Scholar] [CrossRef]
- Sethi, S.; Dhinojwala, A. Superhydrophobic Conductive Carbon Nanotube Coatings for Steel. Langmuir 2009, 25, 4311–4313. [Google Scholar] [CrossRef] [PubMed]
- Francesco De, N.; Paola, C.; Manuela, S.; Francesca, N.; Ilaria, C.; Maurizio De, C.; De Nicola, F.; Castrucci, P.; Scarselli, M.; Nanni, F.; et al. Super-Hydrophobic Multi-Walled Carbon Nanotube Coatings for Stainless Steel. Nanotechnology 2015, 26, 145701. [Google Scholar] [CrossRef]
- Suárez-Martínez, R.; Ocampo-Macias, T.; Lara-Romero, J.; Lemus-Ruiz, J.; Jiménez-Alemán, O.; Chiñas-Castillo, F.; Sagaro-Zamora, R.; Jiménez-Sandoval, S.; Paraguay-Delgado, F. Synthesis and Tribological Performance of Carbon Nanostructures Formed on AISI 316 Stainless Steel Substrates. Tribol. Lett. 2016, 64, 36. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.L. A Simple Thermal CVD Method for Carbon Nanotube Synthesis on Stainless Steel 304 without the Addition of an External Catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Abdulrahaman, M.A.; Abubakre, O.K.; Abdulkareem, S.A.; Tijani, J.O.; Aliyu, A.; Afolabi, A.S. Effect of Coating Mild Steel with CNTs on Its Mechanical Properties and Corrosion Behaviour in Acidic Medium. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 15016. [Google Scholar] [CrossRef]
- Pathak, S.; Lim, E.J.; Pour Shahid Saeed Abadi, P.; Graham, S.; Cola, B.A.; Greer, J.R. Higher Recovery and Better Energy Dissipation at Faster Strain Rates in Carbon Nanotube Bundles: An in-Situ Study. ACS Nano 2012, 6, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Raney, J.R.; De Nardo, L.; Craig, A.E.; Daraio, C. Synthesis and Characterization of Carbon Nanotube-Polymer Multilayer Structures. ACS Nano 2011, 5, 7713–7721. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.D.; Wang, X.; Zhao, H.; Zhu, Y.T. Tuning the Compressive Mechanical Properties of Carbon Nanotube Foam. Carbon 2011, 49, 2834–2841. [Google Scholar] [CrossRef]
- Xu, M.; Futaba, D.N.; Yumura, M.; Hata, K. Alignment Control of Carbon Nanotube Forest from Random to Nearly Perfectly Aligned by Utilizing the Crowding Effect. ACS Nano 2012, 6, 5837–5844. [Google Scholar] [CrossRef] [PubMed]
- Bedewy, M.; Meshot, E.R.; Reinker, M.J.; Hart, A.J. Population Growth Dynamics of Carbon Nanotubes. ACS Nano 2011, 5, 8974–8989. [Google Scholar] [CrossRef] [PubMed]
- Roumeli, E.; Pavlidou, E.; Avgeropoulos, A.; Vourlias, G.; Bikiaris, D.N.D.; Chrissafis, K. Factors Controlling the Enhanced Mechanical and Thermal Properties of Nanodiamond-Reinforced Cross-Linked High Density Polyethylene. J. Phys. Chem. B 2014, 118, 11341–11352. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Flahaut, E.; Peigney, A. The Weight and Density of Carbon Nanotubes versus the Number of Walls and Diameter. Carbon 2010, 48, 2994–2996. [Google Scholar] [CrossRef]
- Felekis, D.; Vogler, H.; Mecja, G.; Muntwyler, S.; Nestorova, A.; Huang, T.; Sakar, M.S.; Grossniklaus, U.; Nelson, B.J. Real-Time Automated Characterization of 3D Morphology and Mechanics of Developing Plant Cells. Int. J. Rob. Res. 2015, 34, 1136–1146. [Google Scholar] [CrossRef]
- Ashby, M.F. Material Property Charts. In Materials Selection in Mechanical Design, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2011; Chapter 4; pp. 57–96. [Google Scholar]
- Chapman, B.N. Thin-Film Adhesion. J. Vac. Sci. Technol. 1974, 11, 106–113. [Google Scholar] [CrossRef]
- Kleinbichler, A.; Pfeifenberger, M.J.; Zechner, J.; Moody, N.R.; Bahr, D.F.; Cordill, M.J. New Insights into Nanoindentation-Based Adhesion Testing. JOM 2017, 69, 2237–2245. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, P.A.; Hintermann, H.E. Adhesion of TiC and Ti(C,N) Coatings on Steel. J. Vac. Sci. Technol. A 1985, 3, 2394–2400. [Google Scholar] [CrossRef]
- Akono, A.T.; Reis, P.M.; Ulm, F.J. Scratching as a Fracture Process: From Butter to Steel. Phys. Rev. Lett. 2011, 106, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, I.; Lahiri, D.; Jin, S.; Agarwal, A.; Choi, W. Carbon Nanotubes: How Strong Is Their Bond with the Substrate? ACS Nano 2011, 5, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Ageev, O.A.; Blinov, Y.F.; Il’ina, M.V.; Il’in, O.I.; Smirnov, V.A.; Tsukanova, O.G. Study of Adhesion of Vertically Aligned Carbon Nanotubes to a Substrate by Atomic-Force Microscopy. Phys. Solid State 2016, 58, 309–314. [Google Scholar] [CrossRef]
- Yu, M.; Funke, H.H.; Falconer, J.L.; Noble, R.D. High Density, Vertically-Aligned Carbon Nanotube Membranes. Nano Lett. 2009, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Seki, Y.; Kimura, H.; Sakurai, S.; Yumura, M.; Hata, K.; Futaba, D.N. Diameter Control of Single-Walled Carbon Nanotube Forests from 1.3-3.0 Nm by Arc Plasma Deposition. Sci. Rep. 2014, 4, 3804. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Lee, Y.T.; Park, J.; Ryu, H.; Lee, H.J.; Choi, S.Y.; Choo, J. Dependence of the Vertically Aligned Growth of Carbon Nanotubes on the Catalysts. J. Phys. Chem. B 2002, 106, 9286–9290. [Google Scholar] [CrossRef]
- De Los Arcos, T.; Garnier, M.G.; Seo, J.W.; Oelhafen, P.; Thommen, V.; Mathys, D. The Influence of Catalyst Chemical State and Morphology on Carbon Nanotube Growth. J. Phys. Chem. B 2004, 108, 7728–7734. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current Understanding of the Growth of Carbon Nanotubes in Catalytic Chemical Vapour Deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Gohier, A.; Ewels, C.P.; Minea, T.M.; Djouadi, M.A. Carbon Nanotube Growth Mechanism Switches from Tip- to Base-Growth with Decreasing Catalyst Particle Size. Carbon 2008, 46, 1331–1338. [Google Scholar] [CrossRef]
- Nessim, G.D.; Hart, A.J.; Kim, J.S.; Acquaviva, D.; Oh, J.; Morgan, C.D.; Seita, M.; Leib, J.S.; Thompson, C.V. Tuning of Vertically-Aligned Carbon Nanotube Diameter and Areal Density through Catalyst Pre-Treatment. Nano Lett. 2008, 8, 3587–3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raney, J.R.; Fraternali, F.; Daraio, C. Rate-Independent Dissipation and Loading Direction Effects in Compressed Carbon Nanotube Arrays. Nanotechnology 2013, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Moisala, A.; Nasibulin, A.G.; Kauppinen, E.I. The Role of Metal Nanoparticles in the Catalytic Production of Single-Walled Carbon Nanotubes—A Review. J. Phys. Condens. Matter 2003, 15, S3011–S3035. [Google Scholar] [CrossRef]
- Meshot, E.R.; Zwissler, D.W.; Bui, N.; Kuykendall, T.R.; Wang, C.; Hexemer, A.; Wu, K.J.J.; Fornasiero, F. Quantifying the Hierarchical Order in Self-Aligned Carbon Nanotubes from Atomic to Micrometer Scale. ACS Nano 2017, 11, 5405–5416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, F.; Zhang, X.; Liang, L.; Yang, X.; Li, Q.; Zhang, X. Vibration Damping of Carbon Nanotube Assembly Materials. Adv. Eng. Mater. 2018, 20, 1700647. [Google Scholar] [CrossRef]
- Won, Y.; Gao, Y.; Panzer, M.A.; Xiang, R.; Maruyama, S.; Kenny, T.W.; Cai, W.; Goodson, K.E. Zipping, Entanglement, and the Elastic Modulus of Aligned Single-Walled Carbon Nanotube Films. Proc. Natl. Acad. Sci. USA 2013, 110, 20426–20430. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent Progress in Superhydrophobic Coatings Used for Steel Protection: A Review. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Bico, J.; Teo, K.B.K.; Chhowalla, M.; Amaratunga, G.A.J.; Milne, W.I.; McKinley, G.H.; Gleason, K.K. Superhydrophobic Carbon Nanotube Forests. Nano Lett. 2003, 3, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the Surface Roughness on Sliding Angles of Water Droplets on Superhydrophobic Surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]





| Steel sample | Cr (wt%) | Ni (wt%) | Mo (wt%) | Cu (wt%) | C (wt%) | Surface roughness (µm) | Surface energy, γs (mJ/m2) |
|---|---|---|---|---|---|---|---|
| S1 | 1.9–2.6 | <0.3 | 0.05–0.15 | 0.2 | 35.4 | ||
| S2 | 8–9.5 | <0.4 | 0.08–0.12 | 0.13 | 30.7 | ||
| S3 | 11–12.5 | 2.2–3 | 1.3–1.9 | 0.1–0.14 | 0.36 | 53.7 | |
| S4 | 13.5–16 | 4–6 | 1.2–2 | 1–2 | 0.07 | 0.85 | 44.8 |
| Substrate Used | Average Water Contact Angle (Degrees) |
|---|---|
| S1 | 150.6 ± 10.8 |
| S2 | 161.5 ± 4.4 |
| S3 | 153.6 ± 5.8 |
| S4 | 144.8 ± 0.3 |
| Si | 133.2 ± 5.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumeli, E.; Diamantopoulou, M.; Serra-Garcia, M.; Johanns, P.; Parcianello, G.; Daraio, C. Characterization of Vertically Aligned Carbon Nanotube Forests Grown on Stainless Steel Surfaces. Nanomaterials 2019, 9, 444. https://doi.org/10.3390/nano9030444
Roumeli E, Diamantopoulou M, Serra-Garcia M, Johanns P, Parcianello G, Daraio C. Characterization of Vertically Aligned Carbon Nanotube Forests Grown on Stainless Steel Surfaces. Nanomaterials. 2019; 9(3):444. https://doi.org/10.3390/nano9030444
Chicago/Turabian StyleRoumeli, Eleftheria, Marianna Diamantopoulou, Marc Serra-Garcia, Paul Johanns, Giulio Parcianello, and Chiara Daraio. 2019. "Characterization of Vertically Aligned Carbon Nanotube Forests Grown on Stainless Steel Surfaces" Nanomaterials 9, no. 3: 444. https://doi.org/10.3390/nano9030444





