Synthesis Procedure of Highly Densely Packed Carbon Nanotube Forests on TiN
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates
2.2. Contact Angle Measurements and Surface Free Energy Calculations of the Different Materials
2.3. Growth of Vertically Aligned SWNTs
2.4. Characterization Methods
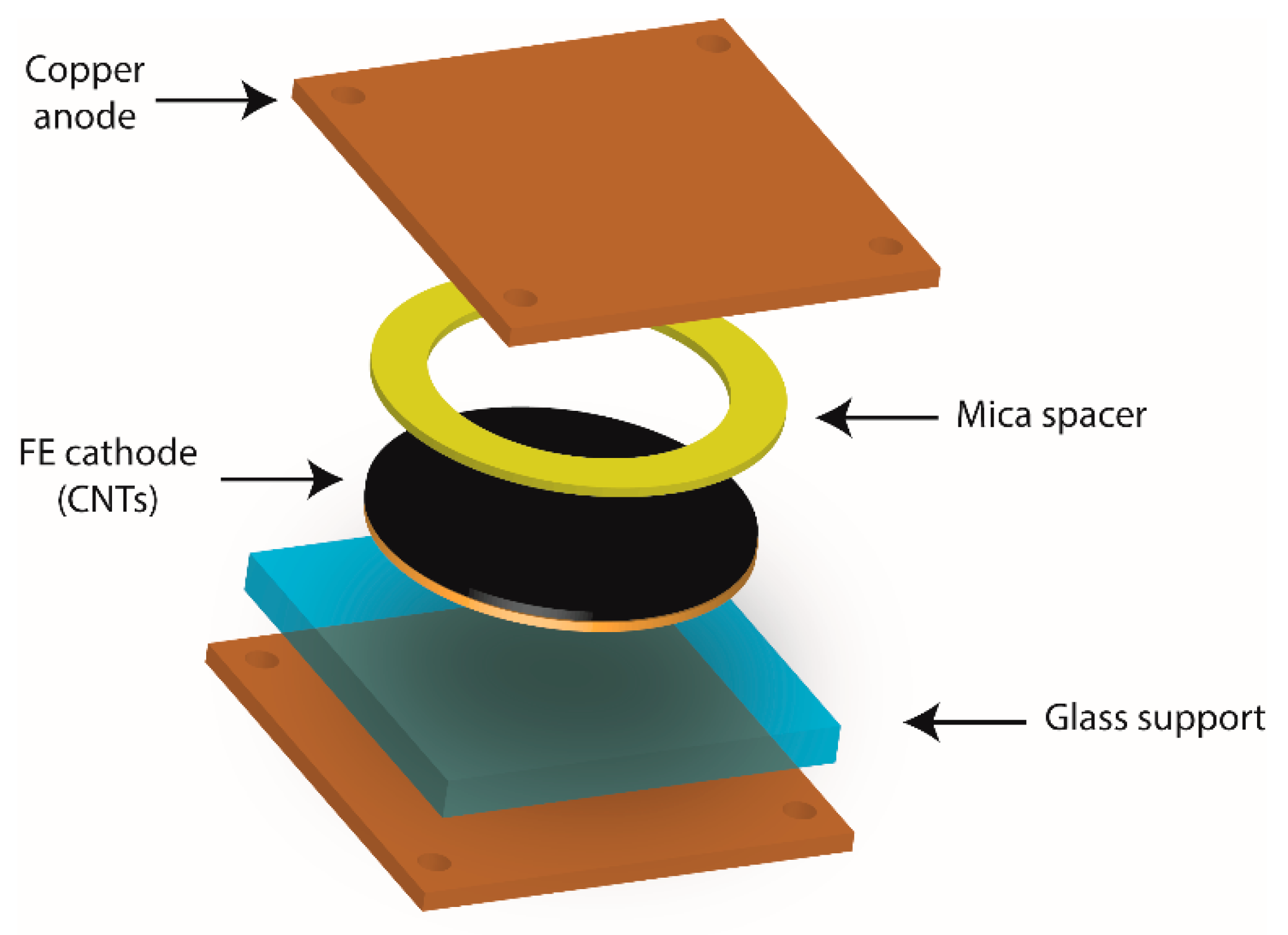
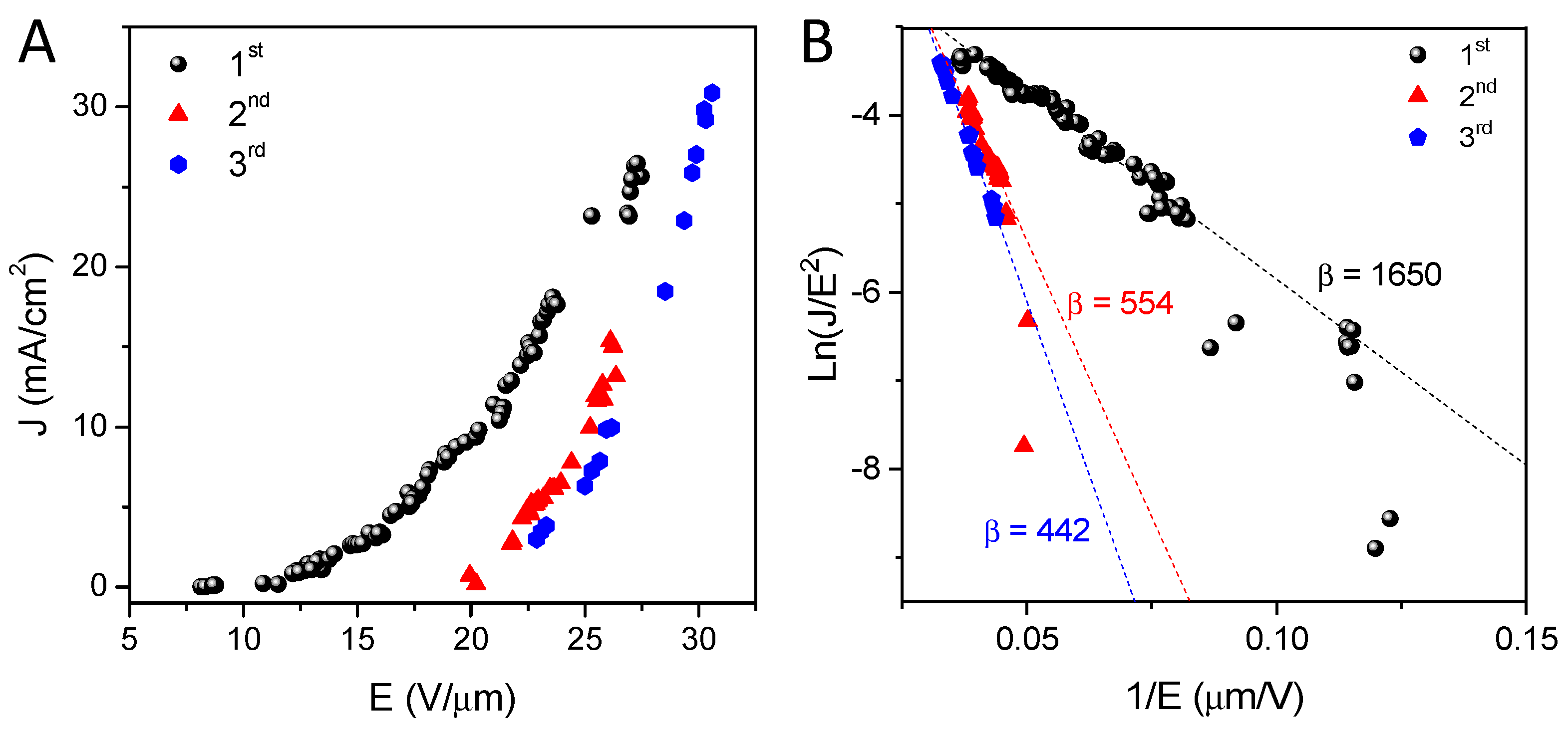
2.5. Field Emission Measurements
3. Results
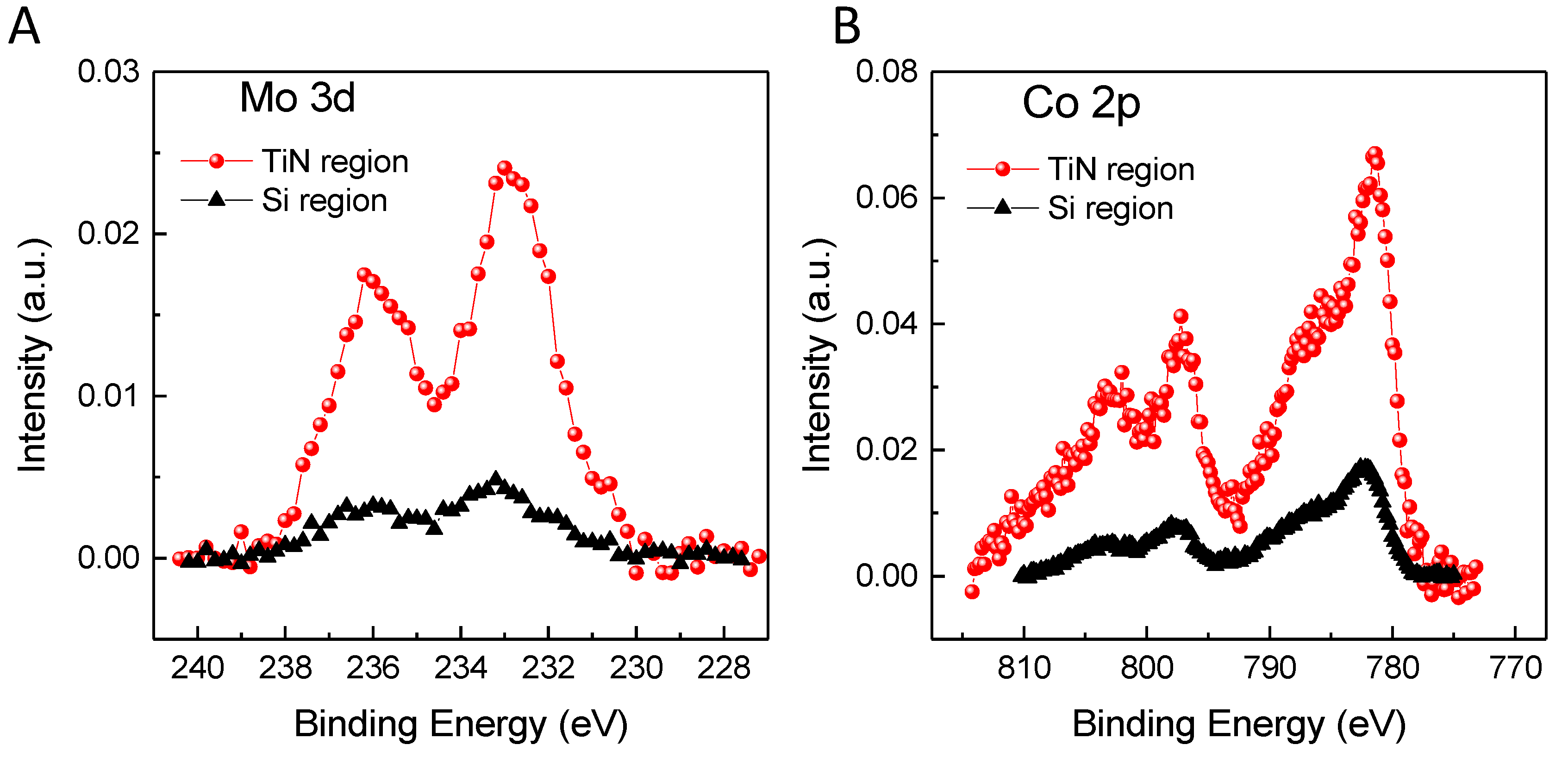
3.1. Deposition of the Catalysts by Dip-Coating
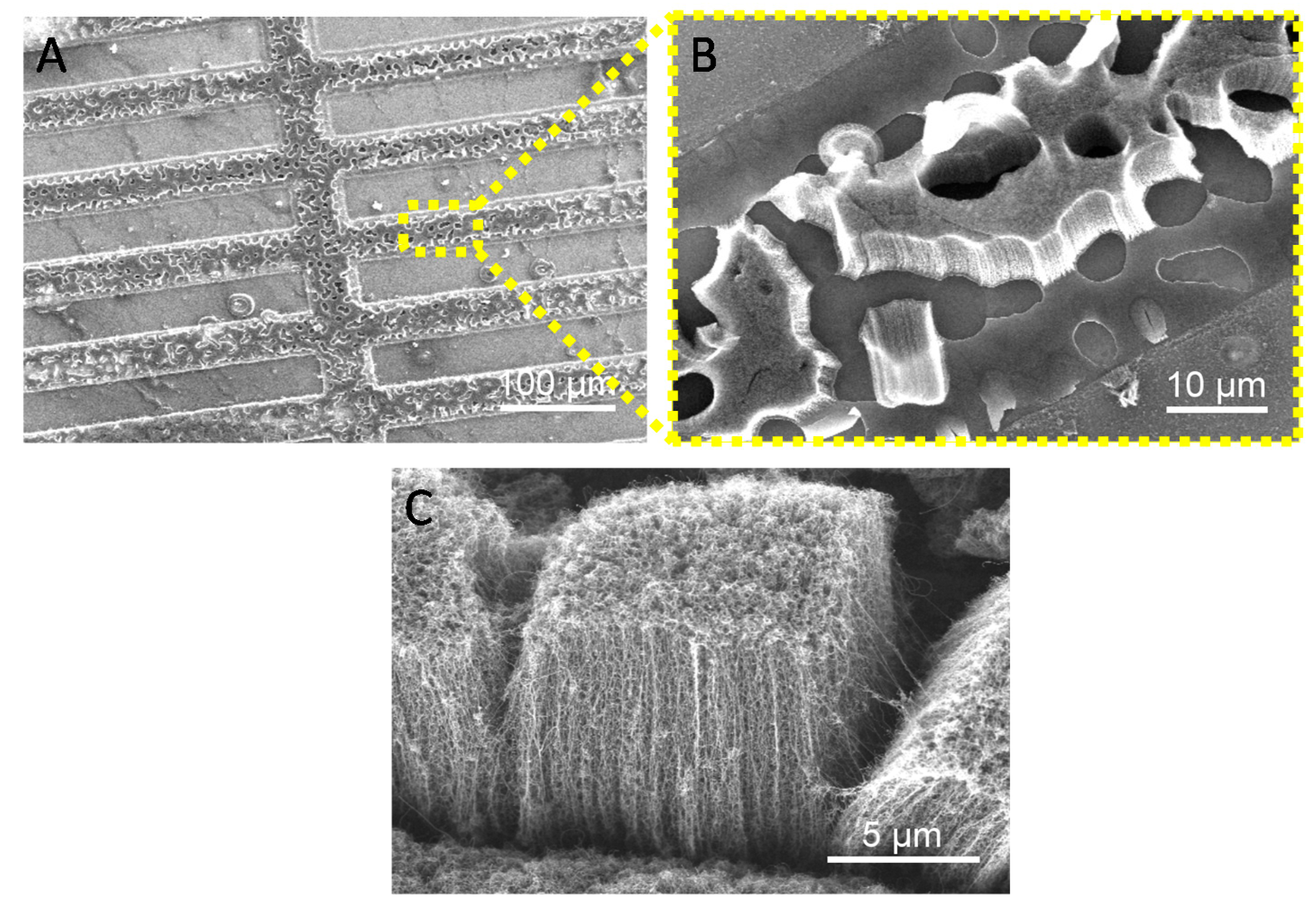
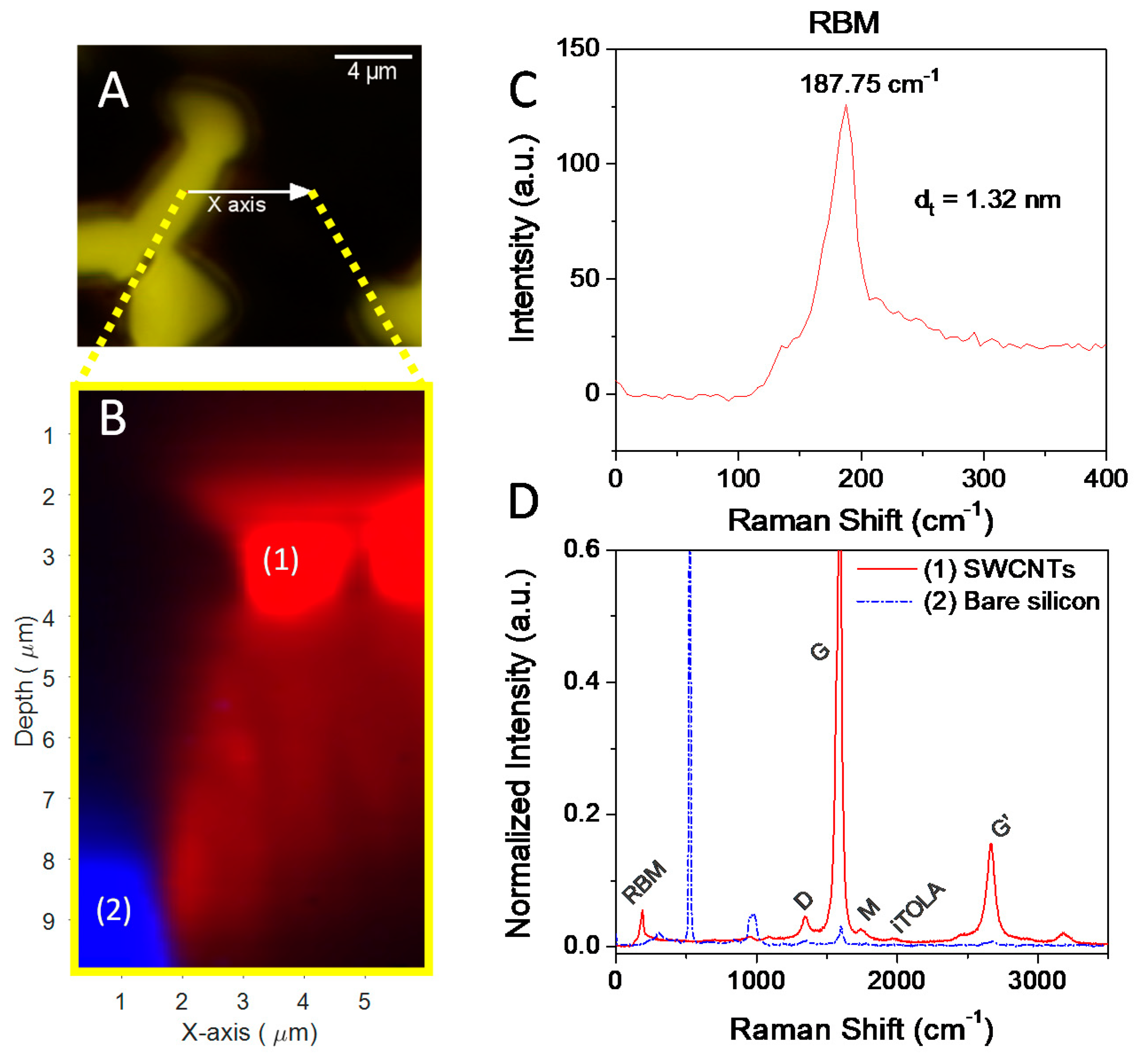
3.2. Growth of SWNTs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who should be given the credit for the discovery of carbon nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Das, R.; Tuhi, S.D. Carbon Nanotubes Synthesis. In Carbon Nanotubes for Clean Water, Carbon Nanostructures; Das, R., Ed.; Springer International Publishing AG, Springer Nature: Basel, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kaushik, B.K.; Majumder, M.K. Carbon Nanotube: Properties and Applications. In Carbon Nanotube Based VLSI Interconnects; Springer Briefs in Applied Sciences and Technology; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Lu, W.; Qu, L.T.; Henry, K.; Dai, L.M. High performance electrochemical capacitors from aligned carbon nanotube electrodes and ionic liquid electrolytes. J. Power Sources 2009, 189, 1270–1277. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Waarbeek, R.F.; Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional Fibers of Carbon Nanotubes with Ultrahigh Conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef]
- Perea-Lopez, N.; Rebollo-Plata, B.; Briones-Leon, J.A.; Morelos-Gomez, A.; Hernandez-Cruz, D.; Hirata, G.A.; Meunier, V.; Botello-Mendez, A.R.; Charlier, J.C.; Maruyama, B.; et al. Millimeter-Long Carbon Nanotubes: Outstanding Electron-Emitting Sources. ACS Nano 2011, 5, 5072–5077. [Google Scholar] [CrossRef]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Titova, L.V.; Pint, C.L.; Zhang, Q.; Hauge, R.H.; Kono, J.; Hegmann, F.A. Generation of Terahertz Radiation by Optical Excitation of Aligned Carbon Nanotubes. Nano Lett. 2015, 15, 3267–3272. [Google Scholar] [CrossRef]
- Kang, S.J.; Kocabas, C.; Ozel, T.; Shim, M.; Pimparkar, N.; Alam, M.A.; Rotkin, S.V.; Rogers, J.A. High-performance electronics using dense, perfectly aligned arrays of single-walled carbon nanotubes. Nat. Nanotechnol. 2007, 2, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, D.N.; Morrill, N.B.; Aten, Q.; Turner, B.W.; Jensen, B.D.; Howell, L.L.; Vanfleet, R.R.; Davis, R.C. Carbon Nanotubes as a Framework for High-Aspect-Ratio MEMS Fabrication. J. Microelectromech. Syst. 2010, 19, 75–82. [Google Scholar] [CrossRef]
- Volder, M.L.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Pápa, Z.; Kecsenovity, E.; Fejes, D.; Budai, J.; Toth, Z.; Hernadi, K. Height and diameter dependence of carbon nanotube forests on the porosity and thickness of catalytic layers. Appl. Surf. Sci. 2018, 428, 885–894. [Google Scholar] [CrossRef]
- Sakurai, S.; Nishino, H.; Futaba, D.N.; Yasuda, S.; Maigne, A.; Matsuo, Y.; Nakamura, E.; Yumura, M.; Hata, K. Role of subsurface diffusion and ostwald ripening in catalyst formation for SWNT forest growth. J. Am. Chem. Soc. 2011, 134, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Sugime, H.; Ushiyama, T.; Nishimura, K.; Ohno, Y.; Noda, S. An interdigitated electrode with dense carbon nanotube forests on conductive supports for electrochemical biosensors. Analyst 2018, 143, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Esconjauregui, S.; Robertson, A.W.; Guo, Y.; Hallam, T.; Sugime, H.; Zhong, G.; Duesberg, G.S.; Robertson, J. Growth of high-density carbon nanotube forests on conductive TiSiN supports. Appl. Phys. Lett. 2015, 106, 083108. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Garcia-Gancedo, L.; Chen, C.; Zhu, X.R.; Xie, H.Q.; Flewitt, A.J.; Milne, W.I. Enzyme-free glucose biosensor based on low density CNT forest grown directly on a Si/SiO2 substrate. Sens. Actuators B Chem. 2013, 178, 586–592. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, J.; Sugime, H.; Rao, R.; Zhao, J.; Liu, D.; Harutyunyan, A.; Robertson, J. Growth of high quality, high density single-walled carbon nanotube forests on copper foils. Carbon 2016, 98, 624–632. [Google Scholar] [CrossRef]
- Kang, L.X.; Li, D.; Yong, Z.Z.; Zhang, X.H.; Li, Q. Growth of Aligned Carbon Nanotubes and Their Applications, Industrial Applications of Carbon Nanotubes. In Growth of Aligned Carbon Nanotubes and Their Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 381–403. [Google Scholar] [CrossRef]
- Ghaemi, F.; Ali, M.; Yunus, R.; Othman, R.N. Synthesis of Carbon Nanomaterials Using Catalytic Chemical Vapor Deposition Technique. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–27. [Google Scholar] [CrossRef]
- Kukovecz, A.; Konya, Z.; Nagaraju, N.; Willems, I.; Tamasi, A.; Fonseca, A.; Nagy, J.B.; Kiricsi, I. Catalytic synthesis of carbon nanotubes over Co, Fe and Ni containing conventional and sol-gel silica-aluminas. Phys. Chem. Chem. Phys. 2000, 2, 3071–3076. [Google Scholar] [CrossRef]
- Mattevi, C.; Wirth, T.; Hofmann, S.; Blume, R.; Cantoro, M.; Ducati, C.; Cepek, C.; Knop-Gericke, A.; Milne, S.; Castellarin-Cudia, C.; et al. In-situ X-ray Photoelectron Spectroscopy Study of Catalyst−Support Interactions and Growth of Carbon Nanotube Forests. J. Phys. Chem. C 2008, 112, 12207–12213. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Xie, R.; Guo, Y.; Pfaendler, S.M.-L.; Fouquet, M.; Gillen, R.; Cepek, C.; Castellarin-Cudia, C.; Eslava, S.; Robertson, J. Electrical conduction of carbon nanotube forests through sub-nanometric films of alumina. Appl. Phys. Lett. 2013, 102, 113109. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Bhardwaj, S.; Yang, J.; Castellarin-Cudia, C.; Xie, R.; D’Arsie, L.; Makaryan, T.; Sugime, H.; Eslava, S.; Cepek, C.; et al. Carbon nanotube growth on conductors: Influence of the support structure and catalyst thickness. Carbon 2014, 73, 13–24. [Google Scholar] [CrossRef]
- Sugime, H.; Esconjauregui, S.; Yang, J.; Arsie, L.; Oliver, R.A.; Bhardwaj, S.; Cepek, C.; Robertson, J. Low temperature growth of ultra-high mass density carbon nanotube forests on conductive supports. Appl. Phys. Lett. 2013, 103, 073116. [Google Scholar] [CrossRef]
- Ahmad, M.; Anguita, J.V.; Stolojan, V.; Corless, T.; Chen, J.-S.; Carey, J.D.; Silva, S.R.P. High Quality Carbon Nanotubes on Conductive Substrates Grown at Low Temperatures. Adv. Funct. Mater. 2015, 25, 4419–4429. [Google Scholar] [CrossRef]
- Amama, P.B.; Pint, C.L.; McJilton, L.; Kim, S.M.; Stach, E.A.; Murray, P.T.; Hauge, R.H.; Maruyama, B. Role of Water in Super Growth of Single-Walled Carbon Nanotube Carpets. Nano Lett. 2009, 9, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, J.; Polsen, E.S.; Oliver, C.R.; Zhao, Y.; Meshot, E.R.; Barclay, M.; Fairbrother, D.H.; Hart, A.J.; Plata, D.L. Oxygen-promoted catalyst sintering influences number density, alignment, and wall number of vertically aligned carbon nanotubes. Nanoscale 2017, 9, 5222–5233. [Google Scholar] [CrossRef] [PubMed]
- Avraham, E.S.; Westover, A.S.; Itzhak, A.; Shani, L.; Mor, V.; Girshevitz, O.; Pint, C.L.; Nessim, G.D. Patterned growth of carbon nanotube forests using Cu and Cu/Ag thin film reservoirs as growth inhibitors. Carbon 2018, 130, 273–280. [Google Scholar] [CrossRef]
- Lahiri, I.; Oh, S.-W.; Hwang, J.Y.; Cho, S.; Sun, Y.-K.; Banerjee, R.; Choi, W. High capacity and excellent stability of lithium ion battery anode using interface-controlled binder-free multiwall carbon nanotubes grown on copper. ACS Nano 2010, 4, 3440–3446. [Google Scholar] [CrossRef]
- Gao, L.; Peng, A.; Wang, Z.Y.; Zhang, H.; Shi, Z.; Gu, Z.; Gao, G.; Ding, B. Growth of aligned carbon nanotube arrays on metallic substrate and its application to supercapacitors. Solid State Commun. 2008, 146, 380–383. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.-L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Fuentes, G.G.; Cáceres, D.; Vergara, I.; Elizalde, E.; Sanz, J.M. Elastic properties of hard TiCxNy films grown by dual ion beam sputtering. Surf. Coat. Technol. 2002, 151–152, 365–369. [Google Scholar] [CrossRef]
- Morant, C.; Campo, T.; Márquez, F.; Domingo, C.; Sanz, J.M.; Elizalde, E. Mo–Co catalyst nanoparticles: Comparative study between TiN and Si surfaces for single-walled carbon nanotube growth. Thin Solid Films 2012, 520, 5232–5238. [Google Scholar] [CrossRef]
- Murakami, Y.; Chiashi, S.; Miyauchi, Y.; Hu, M.; Ogura, M.; Okubo, T.; Maruyama, S. Growth of vertically aligned single-walled carbon nanotube films on quartz substrates and their optical anisotropy. Chem. Phys. Lett. 2004, 385, 298–303. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendung auf die Untersuchung und Veränderung der Oberflächeneigenschaften von Polymeren. Farbe und Lack 1971, 77, 997–1005. [Google Scholar]
- Roumeli, E.; Diamantopoulou, M.; Serra-Garcia, M.; Johanns, P.; Parcianello, G.; Daraio, C. Characterization of vertically aligned carbon nanotube forests grown on stainless steel surfaces. Nanomaterials 2019, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Fowler, R.H.; Nordheim, L. Electron emission in intense electric fields. Proc. R. Soc. A 1928, 119, 173–181. [Google Scholar] [CrossRef]
- Choi, J.-G.; Thompson, L.T. XPS study of as-prepared and reduced molybdenum oxides. Appl. Surf. Sci. 1996, 93, 143–149. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kim, J.W.; Lim, J.S.; Kim, T.J.; Kim, S.D.; Park, S.-J.; Lee, Y.-S. X-ray Photoelectron Spectroscopy Study of Cobalt Supported Multi-walled Carbon Nanotubes Prepared by Different Precursors. Carbon Sci. 2007, 8, 120–126. [Google Scholar] [CrossRef]
- Girardon, J.S.; Quinet, E.; Constant, A.G.; Chernavskii, P.A.; Gengembre, L.; Khodakove. Cobalt dispersion, reducibility, and surface sites in promoted silica-supported Fischer-Tropsch catalysts. J. Catal. 2007, 248, 143–157. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Abel, E.W. Effect of temperature on the surface free energy of amorphous carbon films. J. Colloid Interface Sci. 2004, 280, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R.; Hafner, J.H.; Lieber, C.M.; Hunter, M.; McClure, T.; Dresselhaus, G.; Dresselhaus, M.S. Structural (n,m) determination of isolated single-wall carbon nanotubes by resonant Raman scattering. Phys. Rev. Lett. 2001, 86, 1118–1121. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Yeow, J.T.W. Nanotube field electron emission: Principles, development, and applications. Nanotechnology 2015, 26, 242001. [Google Scholar] [CrossRef]
- Su, W.S.; Leung, T.C.; Chan, C.T. Work function of single-walled and multiwalled carbon nanotubes: First-principles study. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 2–9. [Google Scholar] [CrossRef]
- Saito, Y.; Okuda, M.; Tomita, M.; Hayashi, T. Extrusion of single-wall carbon nanotubes via formation of small particles condensed near an arc evaporation source. Chem. Phys. Lett. 1995, 236, 419–426. [Google Scholar] [CrossRef]
- Morassutto, M.; Tiggelaar, R.M.; Smithers, M.A.; Gardenier, J.G.E. Vertically aligned carbon nanotube field emitter arrays with Ohmic base contact to silicon by Fe-catalyzed chemical vapor deposition. Mater. Today Commun. 2016, 7, 89–100. [Google Scholar] [CrossRef]
- Kleshch, V.I.; Smolnikova, E.A.; Orekhov, A.S.; Kalvas, T.; Tarvainen, O.; Kauppinen, J.; Nuottajärvi, A.; Koivisto, H.; Janhunen, P.; Obraztsov, A.N. Nano-graphite cold cathodes for electric solar wind sail. Carbon 2015, 81, 132–136. [Google Scholar] [CrossRef]




| Substrate | θ (Water) | θ (Diiodomethane) | θ (Ethylene Glycol) | SFE 1 (mJ m−2) |
|---|---|---|---|---|
| Thermal SiO2 | 22.0° | 47.2° | 25.7° | 64.7 |
| Native-SiO2 | 43.9° | 43.3° | 21.3° | 56.8 |
| TiO2 | 52.7° | 45.9° | 45.8° | 48.1 |
| TiN | 61.6° | - | 48.2° | 40.6 |
| Sputtered-Si | 69.7° | 45.5° | 37.0° | 39.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campo, T.; Pinilla, S.; Gálvez, S.; Sanz, J.M.; Márquez, F.; Morant, C. Synthesis Procedure of Highly Densely Packed Carbon Nanotube Forests on TiN. Nanomaterials 2019, 9, 571. https://doi.org/10.3390/nano9040571
Campo T, Pinilla S, Gálvez S, Sanz JM, Márquez F, Morant C. Synthesis Procedure of Highly Densely Packed Carbon Nanotube Forests on TiN. Nanomaterials. 2019; 9(4):571. https://doi.org/10.3390/nano9040571
Chicago/Turabian StyleCampo, Teresa, Sergio Pinilla, Santos Gálvez, José María Sanz, Francisco Márquez, and Carmen Morant. 2019. "Synthesis Procedure of Highly Densely Packed Carbon Nanotube Forests on TiN" Nanomaterials 9, no. 4: 571. https://doi.org/10.3390/nano9040571





