Fabrication of Highly Conductive Porous Cellulose/PEDOT:PSS Nanocomposite Paper via Post-Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of cellulose nanofibers (CNFs)
2.3. Preparation of conductive PEDOT:PSS/CNF nanocomposite papers and their post-treatment
2.4. Characterization
2.5. Electrochemical Measurements
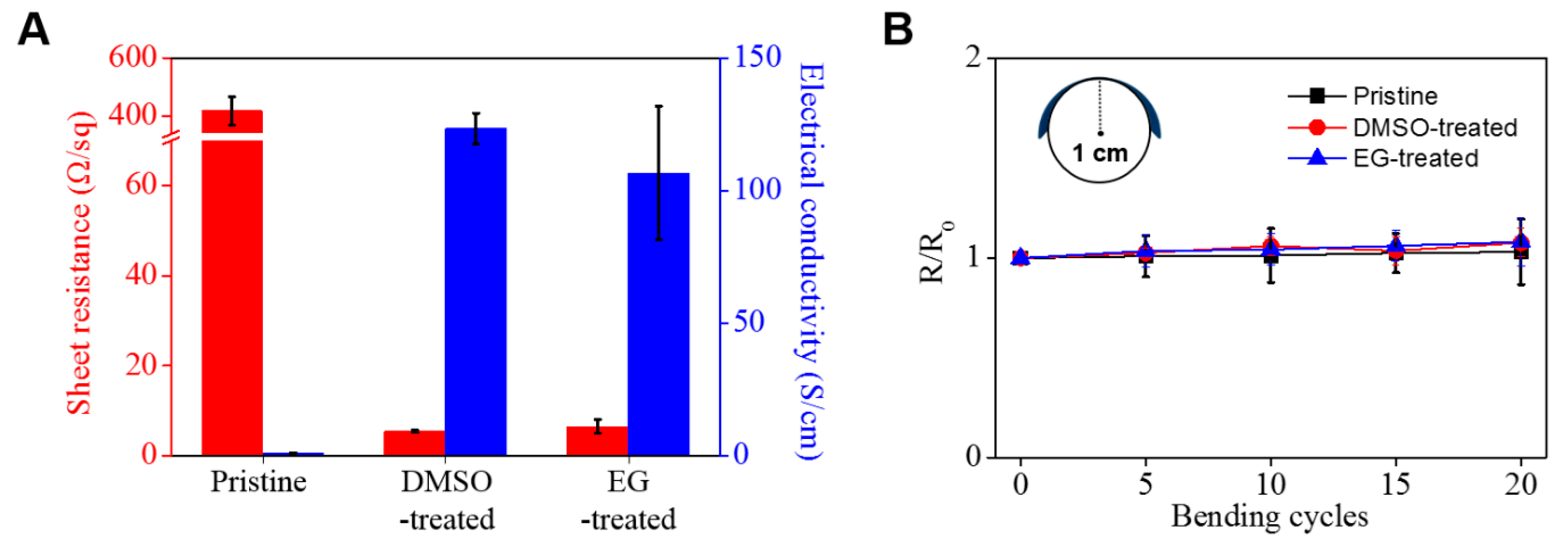
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Kim, J.; Ko, Y.; Shim, K.; Kim, J.H.; You, J. A facile approach for constructing conductive polymer patterns for application in electrochromic devices and flexible microelectrodes. ACS Appl. Mater. Interfaces 2016, 8, 33175–33182. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Lee, S.H.; Cho, W.; Choi, H.H.; Kim, F.S.; Kim, J.H. Effects of one- and two-dimensional carbon hybridization of PEDOT:PSS on the power factor of polymer thermoelectric energy conversion devices. J. Mater. Chem. A 2015, 3, 6526–6533. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Chung, M.H.; Kim, J.; Kim, J.H. A one-step roll-to-roll process of stable AgNW/PEDOT:PSS solution using imidazole as a mild base for highly conductive and transparent films: Optimizations and mechanisms. J. Mater. Chem. C 2015, 3, 5859–5868. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) (PEDOT:PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Kim, J.; Kim, J.H. Highly reliable AgNW/PEDOT:PSS hybrid films: Efficient methods for enhancing transparency and lowering resistance and haziness. J. Mater. Chem. C 2014, 2, 5636–5643. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Tang, J.; Kobayashi, N.; Kim, J.; Ide, Y.; Tominaka, S.; Kim, J.H.; Yamauchi, Y. Ultrahigh performance supercapacitors utilizing core–shell nanoarchitectures from a metal–organic framework-derived nanoporous carbon and a conducting polymer. Chem. Sci. 2016, 7, 5704–5713. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, C.-H.; Kim, Y.; Kim, N.; Lee, W.-J.; Lee, E.-H.; Kim, D.; Park, S.; Lee, K.; Rivnay, J.; et al. Influence of PEDOT:PSS crystallinity and composition on electrochemical transistor performance and long-term stability. Nat. Commun. 2018, 9, 3858. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, J.; Jeong, H.Y.; Kwon, G.; Kim, D.; Ku, M.; Yang, J.; Yamauchi, Y.; Kim, H.-Y.; Lee, C.; et al. Antibacterial poly (3,4-ethylenedioxythiophene):poly(styrene-sulfonate)/agarose nanocomposite hydrogels with thermo-processability and self-healing. Carbohydr. Polym. 2019, 203, 26–34. [Google Scholar] [CrossRef]
- Stritesky, S.; Markova, A.; Vitecek, J.; Safarikova, E.; Hrabal, M.; Kubac, L.; Kubala, L.; Weiter, M.; Vala, M. Printing inks of electroactive polymer PEDOT:PSS: The study of biocompatibility, stability, and electrical properties. J. Biomed. Mater. Res. Part A 2018, 106, 1121–1128. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Wang, X.; Ying, W.; Chen, D.; Ma, X.; Zhao, X.; Peng, X. Highly conductive PEDOT:PSS threaded HKUST-1 thin films. Chem. Commun. 2018, 54, 13865–13868. [Google Scholar] [CrossRef]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z. Highly conductive and transparent PEDOT:PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater. 2012, 22, 421–428. [Google Scholar] [CrossRef]
- Dimitriev, O.P.; Grinko, D.A.; Noskov, Y.V.; Ogurtsov, N.A.; Pud, A.A. PEDOT:PSS films-Effect of organic solvent additives and annealing on the film conductivity. Synth. Met. 2009, 159, 2237–2239. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.-W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Q.; Chen, J.; Yu, X.; Huang, L.; Li, Y.; Li, C.; Shi, G. An ultrahigh-rate electrochemical capacitor based on solution-processed highly conductive PEDOT:PSS films for AC line-filtering. Energy Environ. Sci. 2016, 9, 2005–2010. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, J.; Chen, S.; Zou, J.; Xie, W.; Zeng, X. Highly conductive PEDOT:PSS transparent hole transporting layer with solvent treatment for high performance silicon/organic hybrid solar cells. Nanoscale Res. Lett. 2017, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.-R.; Kim, B.-J.; Lee, K. Highly conductive PEDOT:PSS nanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, D.; Kim, U.J.; You, J. Vacuum-assisted bilayer PEDOT:PSS/cellulose nanofiber composite film for self-standing, flexible, conductive electrodes. Carbohydr. Polym. 2017, 173, 383–391. [Google Scholar] [CrossRef]
- Kim, D.; Ko, Y.; Kwon, G.; Kim, U.-J.; You, J. Micropatterning silver nanowire networks on cellulose nanopaper for transparent paper electronics. ACS Appl. Mater. Interfaces 2018, 10, 38517–38525. [Google Scholar] [CrossRef]
- Kim, I.; Jeon, H.; Kim, D.; You, J.; Kim, D. All-in-one cellulose based triboelectric nanogenerator for electronic paper using simple filtration process. Nano Energy 2018, 53, 975–981. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Ide, Y.; Kim, J.H.; Hossain, M.S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.-W. 3D network of cellulose-based energy storage devices and related emerging applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef]
- Kim, D.; Ko, Y.; Kwon, G.; Choo, Y.-M.; You, J. Low-cost, high-performance plasmonic nanocomposites for hazardous chemical detection using surface enhanced Raman scattering. Sens. Actuators B Chem. 2018, 274, 30–36. [Google Scholar] [CrossRef]
- Kasuga, T.; Isobe, N.; Yagyu, H.; Koga, H.; Nogi, M. Clearly transparent nanopaper from highly concentrated cellulose nanofiber dispersion using dilution and sonication. Nanomaterials 2018, 8, 104. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, S.; Chen, Z.; Wang, S. Fabrication flexible and luminescent nanofibrillated cellulose films with modified SrAl2O4: Eu, Dy phosphors via nanoscale silica and aminosilane. Nanomaterials 2018, 8, 352. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomaki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A 2016, 83, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; An, C.; Fan, S.; Shi, S.; Zhang, R.; Zhang, J.; Li, Q.; Zhang, D.; Hu, X.; Liu, J. Flexible gas sensor based on graphene/ethyl cellulose nanocomposite with ultra-low strain response for volatile organic compounds rapid detection. Nanotechnology 2018, 29, 285501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Carlsson, D.O.; Tammela, P.; Hua, K.; Zhang, P.; Nyholm, L.; Stromme, M. Surface modified nanocellulose fibers yield conducting polymer-based flexible supercapacitors with enhanced capacitances. ACS Nano 2015, 9, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Fan, L.; Gao, L.; Xiong, Y.; Wang, Y.; Ye, Q.; Yu, A.; Dai, H.; Yin, Y.; Cai, J.; et al. Micro-nanostructured polyaniline assembled in cellulose matrix via interfacial polymerization for applications in nerve regeneration. ACS Appl. Mater. Interfaces 2016, 8, 17090–17097. [Google Scholar] [CrossRef]
- Wang, S.; Wei, C.; Gong, Y.; Lv, J.; Yu, C.; Yu, J. Cellulose nanofiber-assisted dispersion of cellulose nanocrystals@polyaniline in water and its conductive films. RSC Adv. 2016, 6, 10168–10174. [Google Scholar] [CrossRef]
- Chen, C.; Bu, X.; Feng, Q.; Li, D. Cellulose nanofiber/carbon nanotube conductive nano-network as a reinforcement template for polydimethylsiloxane nanocomposite. Polymers 2018, 10, 1000. [Google Scholar] [CrossRef]
- Salajkova, M.; Valentini, L.; Zhou, Q.; Berglund, L.A. Tough nanopaper structures based on cellulose nanofibers and carbon nanotubes. Compos. Sci. Technol. 2013, 87, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhao, Z.; Wu, K.; Huang, R.; Liu, T.; Jiang, H.; Chen, F.; Fu, Q. Ultrathin flexible reduced graphene oxide/cellulose nanofiber composite films with strongly anisotropic thermal conductivity and efficient electromagnetic interference shielding. J. Mater. Chem. C 2017, 5, 3748–3756. [Google Scholar] [CrossRef]
- Koga, H.; Nogi, M.; Komoda, N.; Nge, T.T.; Sugahara, T.; Suganuma, K. Uniformly connected conductive networks on cellulose nanofiber paper for transparent paper electronics. NPG Asia Mater. 2014, 6, e93. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Soni, R.; Bhange, S.N.; Kurungot, S. Novel scalable synthesis of highly conducting and robust PEDOT paper for a high performance flexible solid supercapacitor. Energy Environ. Sci. 2015, 8, 1339–1347. [Google Scholar] [CrossRef]
- Valtakari, D.; Liu, J.; Kumar, V.; Xu, C.; Toivakka, M.; Saarinen, J.J. Conductivity of PEDOT:PSS on spin-coated and drop cast nanofibrillar cellulose thin films. Nanoscale Res. Lett. 2015, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, M.; Chen, X.; Xu, F. Facile template synthesis of microfibrillated cellulose/polypyrrole/silver nanoparticles hybrid aerogels with electrical conductive and pressure responsive properties. ACS Sustain. Chem. Eng. 2015, 3, 3346–3354. [Google Scholar] [CrossRef]
- Gou, H.; He, J.; Mo, Z.; Zhao, Z. Ultrasonic preparation of cellulose/Ag/polyaniline conductive composites and its electrical properties. J. Mater. Sci.: Mater. Electron. 2015, 26, 7295–7302. [Google Scholar] [CrossRef]
- Lay, M.; Pelach, M.A.; Pellicer, N.; Tarres, J.A.; Bun, K.N.; Vilaseca, F. Smart nanopaper based on cellulose nanofibers with hybrid PEDOT:PSS/polypyrrole for energy storage devices. Carbohydr. Polym. 2017, 165, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Unuma, T.; Kobayashi, O.; Hamdany, I.F.A.; Kumar, V.; Saarinen, J.J. Terahertz complex conductivity of nanofibrillar cellulose-PEDOT:PSS composite films. Cellulose 2019. [Google Scholar] [CrossRef]
- Young, C.; Wang, J.; Kim, J.; Sugahara, Y.; Henzie, J.; Yamauchi, Y. Controlled chemical vapor deposition for synthesis of nanowire arrays of metal-organic frameworks and their thermal conversion to carbon/metal oxide hybrid materials. Chem. Mater. 2018, 30, 3379–3386. [Google Scholar] [CrossRef]
- Young, C.; Kim, J.; Kaneti, Y.V.; Yamauchi, Y. One-step synthetic strategy of hybrid materials from bimetallic metal-organic frameworks for supercapacitor applications. ACS Appl. Energy. Mater. 2018, 1, 2007–2015. [Google Scholar] [CrossRef]
- Young, C.; Salunkhe, R.R.; Alshehri, S.M.; Ahamad, T.; Huang, Z.; Henzie, J.; Yamauchi, Y. High energy density supercapacitors compared of nickel cobalt oxide nanosheets on nanoporous carbon nanoarchitectures. J. Mater. Chem. A 2017, 5, 11834–11839. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Muller-Meskamp, L.; Leo, K. Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Alonso, E.; Faria, M.; Mohammadkazemi, F.; Resnik, M.; Ferreira, A.; Cordeiro, N. Conductive bacterial cellulose-polyaniline blends: Influence of the matrix and synthesis conditions. Carbohydr. Polym. 2018, 183, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.; Gonzalez, I.; Tarres, J.A.; Pellicer, N.; Bun, K.N.; Vilaseca, F. High electrical and electrochemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose–poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) composites for optoelectronic applications. Carbohydr. Polym. 2015, 127, 86–93. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Q.; Chen, W.; Yi, X.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Li, X.; Yu, H. Highly flexible and conductive cellulose-mediated PEDOT:PSS/MWCNT composite films for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2017, 9, 13213–13222. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Ni, D.; Chen, Y.; Song, H.; Liu, C.; Yang, X.; Cai, K. Free-standing and highly conductive PEDOT nanowire films for high-performance all-solid-state supercapacitors. J. Mater. Chem. A 2019, 7, 1323–1333. [Google Scholar] [CrossRef]
- Lay, M.; Mendez, J.A.; Delgado-Aguilar, M.; Bun, K.N.; Vilaseca, F. Strong and electrically conductive nanopaper from cellulose nanofibers and polypyrrole. Carbohydr. Polym. 2016, 152, 361–369. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.; Kim, J.; Kim, D.; Kwon, G.; Yamauchi, Y.; You, J. Fabrication of Highly Conductive Porous Cellulose/PEDOT:PSS Nanocomposite Paper via Post-Treatment. Nanomaterials 2019, 9, 612. https://doi.org/10.3390/nano9040612
Ko Y, Kim J, Kim D, Kwon G, Yamauchi Y, You J. Fabrication of Highly Conductive Porous Cellulose/PEDOT:PSS Nanocomposite Paper via Post-Treatment. Nanomaterials. 2019; 9(4):612. https://doi.org/10.3390/nano9040612
Chicago/Turabian StyleKo, Youngsang, Jeonghun Kim, Dabum Kim, Goomin Kwon, Yusuke Yamauchi, and Jungmok You. 2019. "Fabrication of Highly Conductive Porous Cellulose/PEDOT:PSS Nanocomposite Paper via Post-Treatment" Nanomaterials 9, no. 4: 612. https://doi.org/10.3390/nano9040612




