Ionic Liquids as Delaminating Agents of Layered Double Hydroxide during In-Situ Synthesis of Poly (Butylene Adipate-co-Terephthalate) Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LDH with Intercalated IL-Anions
2.2. Preparation of PBAT/LDH Nanocomposites
2.3. Characterizations
3. Results and Discussion
3.1. Synthesis of Layered Double Hydroxide with Intercalated Ionic Liquid Anions
3.1.1. FTIR Spectra
3.1.2. XRD Patterns
3.1.3. Thermogravimetric Analysis
3.2. Characterization of PBAT/LDH Nanocomposites
3.2.1. Morphology
3.2.2. Water Vapor and Gas Barrier Properties
3.2.3. Thermal Properties
3.2.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Li, J.; Lai, L.; Wu, L.; Severtson, S.J.; Wang, W.J. Enhancement of Water Vapor Barrier Properties of Biodegradable Poly (butylene adipate-co-terephthalate) Films with Highly Oriented Organomontmorillonite. ACS Sustain. Chem. Eng. 2018, 6, 6654–6662. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Zhao, Q.; Yang, Y.; Xu, J.; Waterhouse, G.I.; Zhang, K.; Li, S.; Jin, P.; Jin, G. Scale-Up Fabrication of Biodegradable Poly (butylene adipate-co-terephthalate)/Organophilic–clay Nanocomposite Films for Potential Packaging Applications. ACS Omega 2018, 3, 1187–1196. [Google Scholar] [CrossRef]
- Someya, Y.; Sugahara, Y.; Shibata, M. Nanocomposites based on poly (butylene adipate-co-terephthalate) and montmorillonite. J. Appl. Polym. Sci. 2005, 95, 386–392. [Google Scholar] [CrossRef]
- Fukushima, K.; Wu, M.H.; Bocchini, S.; Rasyida, A.; Yang, M.C. PBAT based nanocomposites for medical and industrial applications. Mater. Sci. Eng. 2012, 32, 1331–1351. [Google Scholar] [CrossRef]
- Xu, G.; Qin, S.; Yu, J.; Huang, Y.; Zhang, M.; Ruan, W. Effect of migration of layered nanoparticles during melt blending on the phase morphology of poly (ethylene terephthalate)/polyamide 6/montmorillonite ternary nanocomposites. RSC Adv. 2015, 5, 29924–29930. [Google Scholar] [CrossRef]
- Falcão, G.A.; Vitorino, M.B.; Almeida, T.G.; Bardi, M.A.; Carvalho, L.H.; Canedo, E.L. PBAT/organoclay composite films: Preparation and properties. Polym. Bull. 2017, 74, 4423–4436. [Google Scholar] [CrossRef]
- Livi, S.; Lins, L.; Peter, J.; Benes, H.; Kredatusova, J.; Donato, R.; Pruvost, S. Ionic liquids as surfactants for layered double hydroxide fillers: Effect on the final properties of poly (butylene adipate-co-terephthalate). Nanomaterials 2017, 7, 297. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Kao, N.; Hadigheh, S.A.; Bhattacharya, S.N. Influence of graphene nanoplatelet incorporation and dispersion state on thermal, mechanical and electrical properties of biodegradable matrices. J. Mater. Sci. Technol. 2018, 34, 1026–1034. [Google Scholar] [CrossRef]
- Ren, P.G.; Liu, X.H.; Ren, F.; Zhong, GJ.; Ji, X.; Xu, L. Biodegradable graphene oxide nanosheets/poly-(butylene adipate-co-terephthalate) nanocomposite film with enhanced gas and water vapor barrier properties. Polym. Test. 2017, 58, 173–180. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Li, D.; Tang, P.; Leroux, F.; Feng, Y. Micrometer-sized dihydrogenphosphate-intercalated layered double hydroxides: Synthesis, selective infrared absorption properties, and applications as agricultural films. Dalton Trans. 2018, 47, 3144–3154. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, K.; Wu, J.; Ren, G.; Chen, H.; Xu, J. Bio-nanocomposite films reinforced with organo-modified layered double hydroxides: Preparation, morphology and properties. Appl. Clay Sci. 2016, 126, 72–80. [Google Scholar] [CrossRef]
- Hennous, M.; Derriche, Z.; Privas, E.; Navard, P.; Verney, V.; Leroux, F. Lignosulfonate interleaved layered double hydroxide: A novel green organoclay for bio-related polymer. Appl. Clay Sci. 2013, 71, 42–48. [Google Scholar] [CrossRef]
- Morelli, C.L.; Belgacem, N.; Bretas, R.E.; Bras, J. Melt extruded nanocomposites of polybutylene adipate-co-terephthalate (PBAT) with phenylbutyl isocyanate modified cellulose nanocrystals. J. Appl. Polym. Sci. 2016, 133, 43678. [Google Scholar] [CrossRef]
- Mao, N.; Zhou, C.H.; Tong, D.S.; Yu, W.H.; Lin, C.C. Exfoliation of layered double hydroxide solids into functional nanosheets. Appl. Clay Sci. 2017, 144, 60–78. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring hybrid layered double hydroxides for the development of innovative applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Wang, G.A.; Wang, C.C.; Chen, C.Y. The disorderly exfoliated LDHs/PMMA nanocomposite synthesized by in situ bulk polymerization. Polymer 2005, 46, 5065–5074. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Kredatusová, J.; Beneš, H.; Livi, S.; Pop-Georgievski, O.; Ecorchard, P.; Abbrent, S.; Pavlova, E.; Bogdał, D. Influence of ionic liquid-modified LDH on microwave-assisted polymerization of ε-caprolactone. Polymer 2016, 100, 86–94. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Vieira, R.S.; Ferreira, S.O.; Reis, C. Preparation and evaluation of the Ca-Al layered double hydroxide for removal of copper (II), nickel (II), zinc (II), chromium (VI) and phosphate from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 5469–5480. [Google Scholar] [CrossRef]
- Perz, V.; Bleymaier, K.; Sinkel, C.; Kueper, U.; Bonnekessel, M.; Ribitsch, D.; Guebitz, G.M. Data on synthesis of oligomeric and polymeric poly (butylene adipate-co-butylene terephthalate) model substrates for the investigation of enzymatic hydrolysis. Data Brief 2016, 7, 291–298. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Do, D.D. Review of time lag permeation technique as a method for characterisation of porous media and membranes. Adsorption 1997, 3, 283–312. [Google Scholar] [CrossRef]
- Chivrac, F.; Kadlecová, Z.; Pollet, E.; Avérous, L. Aromatic copolyester-based nano-biocomposites: Elaboration, structural characterization and properties. J. Polym. Environ. 2006, 14, 393–401. [Google Scholar] [CrossRef]
- Herrera, R.; Franco, L.; Rodriquez-Galan, A.; Puiggali, J. Characterization and degradation behavior of poly(butyleneadipate-co-terephthalate)s. J. Appl. Polym. Sci. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Witschard, G.; Griffin, C.E. Infrared absorption characteristics of alkyl and aryl substituted phosphonium salts. Spectrochim. Acta 1963, 19, 1905–1910. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Ha, J.U.; Xanthos, M. Novel modifiers for layered double hydroxides and their effects on the properties of polylactic acid composites. Appl. Clay Sci. 2010, 47, 303–310. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ciotta, E.; Ferrari, I.V.; Narducci, R.; Pasquini, L.; Varone, A.; Richetta, M.; Antonaroli, S.; Braglia, M.; Knauth, P.; et al. Layered double hydroxides containing an ionic liquid: Ionic conductivity and use in composite anion exchange membranes. Chem. Electr. Chem. 2018, 5, 2781–2788. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Haeussler, L.; Heinrich, G. Intercalation of Mg–Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Wang, D.Y.; Costa, F.R.; Vyalikh, A.; Leuteritz, A.; Scheler, U.; Jehnichen, D.; Wagenknecht, U.; Haussler, D.; Heinrich, G. One-step synthesis of organic LDH and its comparison with regeneration and anion exchange method. Chem. Mater. 2009, 21, 4490–4497. [Google Scholar] [CrossRef]
- You, Y.; Vance, G.F.; Zhao, H. Selenium adsorption on Mg–Al and Zn–Al layered double hydroxide. Appl. Clay Sci. 2001, 20, 13–25. [Google Scholar] [CrossRef]
- Kameda, T.; Saito, M.; Umetsu, Y. Preparation and characterisation of Mg–Al layered double hydroxides intercalated with 2-naphthalene sulphonate and 2,6-naphthalene disulphonate. Mater. Trans. 2006, 47, 923–930. [Google Scholar] [CrossRef]
- Miyata, S. The Syntheses of Hydrotalcite-Like Compounds and Their Structures and Physico-Chemical Properties—I: The Systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays. Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Soares, B.G.; Ferreira, S.C.; Livi, S. Modification of anionic and cationic clays by zwitterionic imidazolium ionic liquid and their effect on the epoxy-based nanocomposites. Appl. Clay Sci. 2017, 135, 347–354. [Google Scholar] [CrossRef]
- Hou, X.; Bish, D.L.; Wang, S.L.; Johnston, C.T.; Kirkpatrick, R.J. Hydration, expansion, structure, and dynamics of layered double hydroxides. Am. Min. 2003, 88, 167–179. [Google Scholar] [CrossRef]
- Li, Q.; Kirkpatrick, R.J. Organic anions in layered double hydroxides: An experimental investigation of citrate hydrotalcite. Am. Miner. 2007, 92, 397–402. [Google Scholar] [CrossRef]
- Livi, S.; Bugatti, V.; Soares, B.G.; Duchet-Rumeau, J. Structuration of ionic liquids in a poly (butylene-adipate-co-terephthalate) matrix: Its influence on the water vapour permeability and mechanical properties. Green Chem. 2014, 16, 3758–3762. [Google Scholar] [CrossRef]
- Giel, V.; Galajdová, B.; Popelková, D.; Kredatusová, J.; Trchová, M.; Pavlova, E.; Beneš, H.; Válek, R.; Peter, J. Gas transport properties of novel mixed matrix membranes made of titanate nanotubes and PBI or PPO. Desalin. Water Treat 2015, 56, 3285–3293. [Google Scholar] [CrossRef]
- Poláková, L.; Sedláková, Z.; Ecorchard, P.; Pavlova, E.; Peter, J.; Paruzel, B.; Beneš, H. Poly (meth) acrylate nanocomposite membranes containing in situ exfoliated graphene platelets: Synthesis, characterization and gas barrier properties. Eur. Polym. J. 2017, 94, 431–445. [Google Scholar] [CrossRef]
- Strawhecker, K.E.; Manias, E. Structure and properties of poly (vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem. Mater. 2000, 12, 2943–2949. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the barrier properties of polymer-layered silicate nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Chen, J.H.; Yang, M.C. Preparation and characterization of nanocomposite of maleated poly (butylene adipate-co-terephthalate) with organoclay. Mater. Sci. Eng. 2015, 46, 301–308. [Google Scholar] [CrossRef]
- Livi, S.; Sar, G.; Bugatti, V.; Espuche, E.; Duchet-Rumeau, J. Synthesis and physical properties of new layered silicates based on ionic liquids: Improvement of thermal stability, mechanical behaviour and water permeability of PBAT nanocomposites. RSC Adv. 2014, 4, 26452–26461. [Google Scholar] [CrossRef]
- Livi, S.; Gérard, J.F.; Duchet-Rumeau, J. Ionic liquids: Structuration agents in a fluorinated matrix. Chem. Commun. 2011, 47, 3589–3591. [Google Scholar] [CrossRef]






| Ionic Liquid | Chemical Structure | Designation |
|---|---|---|
| Trihexyltetradecylphosphonium bis (2,4,4-trimethylpentyl) phosphinate | 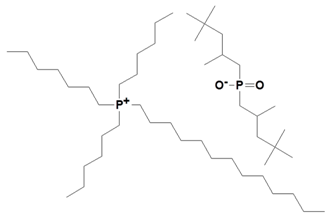 | IL-phosphinate |
| Trihexyltetradecylphosphonium decanoate | 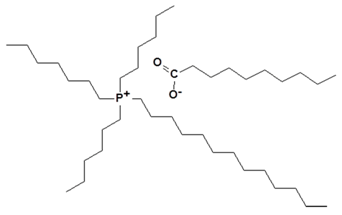 | IL-decanoate |
| Trihexyltetradecylphosphonium bis (2-ethylhexyl) phosphate |  | IL-phosphate |
| Permeability Coefficient (Barrer) 1 | Ideal Selectivity | |||||
|---|---|---|---|---|---|---|
| O2 | CO2 | H2O | CO2/O2 | H2O/O2 | H2O/CO2 | |
| Neat PBAT | 1.55 | 16.9 | 2730 | 10.9 | 1770 | 162 |
| +1.5% pristine LDH | 1.42 | 15.5 | 2660 | 10.9 | 1880 | 172 |
| +1.5% LDH-phosphate | 1.35 | 14.3 | 1570 | 10.6 | 1170 | 110 |
| +1.5% LDH-decanoate | 1.48 | 16.1 | 2010 | 10.9 | 1358 | 125 |
| +1.5% LDH-phosphinate | 1.48 | 16.3 | 1980 | 11.0 | 1338 | 121 |
| +5% pristine LDH | 1.33 | 15.1 | 2550 | 11.4 | 1930 | 169 |
| +5% LDH-phosphate | 1.30 | 14.1 | 2300 | 10.8 | 1770 | 163 |
| +5% LDH-decanoate | 1.21 | 12.5 | 1530 | 10.3 | 1264 | 122 |
| +5% LDH-phosphinate | 1.43 | 14.0 | 1740 | 9.8 | 1217 | 124 |
| Tg [°C] | Tm [°C] | ΔHm [J/g] | Xc [%] | Td5% [°C] | |
|---|---|---|---|---|---|
| Neat PBAT | −40 | 116 | 9.7 | 8 | 354 |
| +1.5% pristine LDH | −39 | 108 | 11.8 | 11 | 351 |
| +1.5% LDH-phosphate | −36 | 111 | 10.1 | 9 | 336 |
| +1.5% LDH-decanoate | −38 | 103 | 11.4 | 10 | 331 |
| +1.5% LDH-phosphinate | −38 | 110 | 10.9 | 10 | 342 |
| +5% pristine LDH | −40 | 113 | 8.3 | 8 | 346 |
| +5% LDH-phosphate | −37 | 102 | 11.3 | 10 | 317 |
| +5% LDH-decanoate | −37 | 99 | 13.6 | 13 | 310 |
| +5% LDH-phosphinate | −35 | 101 | 13.2 | 12 | 324 |
| Young Modulus [MPa] | Tensile Strength [MPa] | Elongation at Break [%] | |
|---|---|---|---|
| Neat PBAT | 76 ± 4 | 5.6 ± 0.3 | 122 ± 23 |
| +1.5% pristine LDH | 92 ± 4 | 5.9 ± 0.4 | 121 ± 18 |
| +1.5% LDH-phosphate | 110 ± 2 | 6.8 ± 0.4 | 133 ± 20 |
| +1.5% LDH-decanoate | 104 ± 4 | 5.0 ± 0.2 | 13 ± 3 |
| +1.5% LDH-phosphinate | 94 ± 2 | 8.7 ± 0.5 | 227 ± 15 |
| +5% pristine LDH | 99 ± 4 | 5.2 ± 0.5 | 29 ± 16 |
| +5% LDH-phosphate | 106 ± 4 | 6.2 ± 0.2 | 20 ± 3 |
| +5% LDH-decanoate | 137 ± 4 | 5.1 ± 0.1 | 10 ± 1 |
| +5% LDH-phosphinate | 112 ± 5 | 8.3 ± 0.6 | 225 ± 43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beneš, H.; Kredatusová, J.; Peter, J.; Livi, S.; Bujok, S.; Pavlova, E.; Hodan, J.; Abbrent, S.; Konefał, M.; Ecorchard, P. Ionic Liquids as Delaminating Agents of Layered Double Hydroxide during In-Situ Synthesis of Poly (Butylene Adipate-co-Terephthalate) Nanocomposites. Nanomaterials 2019, 9, 618. https://doi.org/10.3390/nano9040618
Beneš H, Kredatusová J, Peter J, Livi S, Bujok S, Pavlova E, Hodan J, Abbrent S, Konefał M, Ecorchard P. Ionic Liquids as Delaminating Agents of Layered Double Hydroxide during In-Situ Synthesis of Poly (Butylene Adipate-co-Terephthalate) Nanocomposites. Nanomaterials. 2019; 9(4):618. https://doi.org/10.3390/nano9040618
Chicago/Turabian StyleBeneš, Hynek, Jana Kredatusová, Jakub Peter, Sébastien Livi, Sonia Bujok, Ewa Pavlova, Jiří Hodan, Sabina Abbrent, Magdalena Konefał, and Petra Ecorchard. 2019. "Ionic Liquids as Delaminating Agents of Layered Double Hydroxide during In-Situ Synthesis of Poly (Butylene Adipate-co-Terephthalate) Nanocomposites" Nanomaterials 9, no. 4: 618. https://doi.org/10.3390/nano9040618





