Electrical Properties and Interfacial Issues of HfO2/Ge MIS Capacitors Characterized by the Thickness of La2O3 Interlayer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Bonding States of the Interfaces between the Deposited Films and Ge Substrates
3.2. Microstructures of the Deposited Films on Ge Substrates
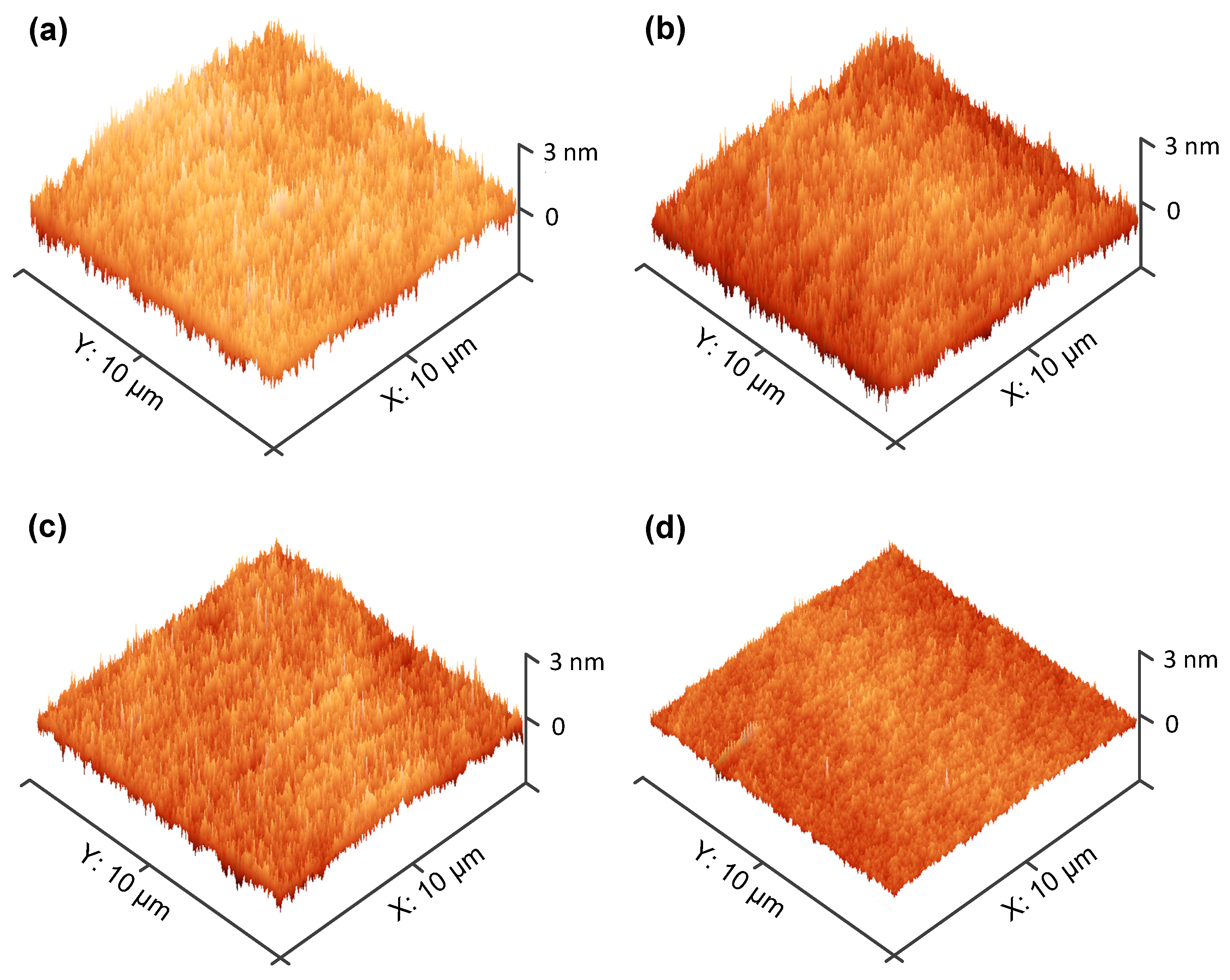
3.3. Surface Morphology of HfO2 and HfO2/La2O3 Gate Stacks on Ge Substrates
3.4. Electrical Performance of Al/HfO2/Ge and Al/HfO2/La2O3/Ge MIS Capacitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, B.H.; Oh, J.; Tseng, H.H.; Jammy, R.; Huff, H. Gate Stack Technology for Nanoscale Devices. Mater. Today 2006, 9, 32–40. [Google Scholar] [CrossRef]
- Kamata, Y. High-K/Ge MOSFETs for Future Nanoelectronics. Mater. Today 2008, 11, 30–38. [Google Scholar] [CrossRef]
- Yi, S.H.; Chang-Liao, K.S.; Wu, T.Y.; Hsu, C.W.; Huang, J.Y. High Performance Ge pMOSFETs with HfO2/Hf-Cap/GeOx Gate Stack and Suitable Post Metal Annealing Treatments. IEEE Electron Device Lett. 2017, 38, 544–547. [Google Scholar] [CrossRef]
- Simoen, E.; Mitard, J.; Hellings, G.; Eneman, G.; DeJaeger, B.; Witters, L.; Vincent, B.; Loo, R.; Delabie, A.; Sioncke, S.; et al. Challenges and Opportunities in Advanced Ge pMOSFETs. Mater. Sci. Semicond. Process. 2012, 15, 588–600. [Google Scholar] [CrossRef]
- Houssa, M.; Chagarov, E.; Kummel, A. Surface Defects and Passivation of Ge and III–V Interfaces. MRS Bull. 2009, 34, 504–513. [Google Scholar] [CrossRef]
- Kouda, M.; Suzuki, T.; Kakushima, K.; Ahmet, P.; Iwai, H.; Yasuda, T. Electrical Properties of CeO2/La2O3 Stacked Gate Dielectrics Fabricated by Chemical Vapor Deposition and Atomic Layer Deposition. Jpn. J. Appl. Phys. 2012, 51, 121101. [Google Scholar]
- Liu, Q.Y.; Fang, Z.B.; Liu, S.Y.; Tan, Y.S.; Chen, J.J. Band Offsets of La2O3 Films on Ge Substrates Grown by Radio Frequency Magnetron Sputtering. Mater. Lett. 2014, 116, 43–45. [Google Scholar] [CrossRef]
- Abermann, S.; Bethge, O.; Henkel, C.; Bertagnolli, E. Atomic Layer Deposition of ZrO2/La2O3 High-K Dielectrics on Germanium Reaching 0.5 Nm Equivalent Oxide Thickness. Appl. Phys. Lett. 2009, 94, 262904. [Google Scholar] [CrossRef]
- Calmels, L.; Coulon, P.E.; Schamm-Chardon, S. Calculated and Experimental Electron Energy-Loss Spectra of La2O3, La(OH)3, and LaOF Nanophases in High Permittivity Lanthanum-Based Oxide Layers. Appl. Phys. Lett. 2011, 98, 243116. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, H.X.; Wang, X.; Wang, Y.T.; Wang, S.L. Improvements on the Interfacial Properties of High-K/Ge MIS Structures by Inserting a La2O3 Passivation Layer. Materials 2018, 11, 2333. [Google Scholar] [CrossRef]
- Eom, D.; Hwang, C.S.; Kim, H.J. Thermal Stability of Stack Structures of Aluminum Nitride and Lanthanum Oxide Thin Films. ECS Trans. 2006, 3, 121–127. [Google Scholar]
- Hausmann, D.M.; Kim, E.; Becker, J.; Gordon, R.G. Atomic Layer Deposition of Hafnium and Zirconium Oxides Using Metal Amide Precursors. Chem. Mater. 2002, 14, 4350–4358. [Google Scholar] [CrossRef]
- Cao, D.; Cheng, X.H.; Yu, Y.H.; Li, X.L.; Liu, C.Z.; Shen, D.S.; Mändl, S. Competitive Si and La Effect in HfO2 Phase Stabilization in Multi-Layer (La2O3)0.08(HfO2) Films. Appl. Phys. Lett. 2013, 103, 081607. [Google Scholar] [CrossRef]
- Mitrovic, I.Z.; Althobaiti, M.; Weerakkody, A.D.; Sedghi, N.; Hall, S.; Dhanak, V.R.; Chalker, P.R.; Henkel, C.; Dentoni, L.E.; Hellström, P.E.; et al. Interface Engineering of Ge Using Thulium Oxide: Band Line-Up Study. Microelectron. Eng. 2013, 109, 204–207. [Google Scholar] [CrossRef]
- Song, J.; Kakushima, K.; Ahmet, P.; Tsutsui, K.; Sugii, N.; Hattori, T.; Iwai, H. Improvement of Interfacial Properties with Interfacial Layer in La2O3/Ge Structure. Microelectron. Eng. 2007, 84, 2336–2339. [Google Scholar] [CrossRef]
- Li, X.F.; Liu, X.J.; Cao, Y.Q.; Li, A.D.; Li, H.; Wu, D. Improved Interfacial and Electrical Properties of Atomic Layer Deposition HfO2 Films on Ge with La2O3 Passivation. Appl. Surf. Sci. 2013, 264, 783–786. [Google Scholar] [CrossRef]
- Kim, H.C.; Woo, S.H.; Lee, J.S.; Kim, H.G.; Kim, Y.C.; Lee, H.R.; Jeon, H.T. The Effects of Annealing Ambient on the Characteristics of La2O3 Films Deposited by RPALD. J. Electrochem. Soc. 2010, 157, H479–H482. [Google Scholar] [CrossRef]
- Mitrovic, I.Z.; Althobaiti, M.; Weerakkody, A.D.; Dhanak, V.R.; Linhart, W.M.; Veal, T.D.; Sedghi, N.; Hall, S.; Chalker, P.R.; Tsoutsou, D.; et al. Ge Interface Engineering Using Ultra-Thin La2O3 and Y2O3 Films: A Study into the Effect of Deposition Temperature. J. Appl. Phys. 2014, 115, 114102. [Google Scholar] [CrossRef]
- Schmeisser, D.; Schnell, R.D.; Bogen, A.; Himpsel, F.J.; Rieger, D.; Landgren, G.; Morar, J.F. Surface Oxidation States of Germanium. Surf. Sci. 1986, 172, 455–465. [Google Scholar] [CrossRef]
- Wilka, G.D.; Wallace, R.M. Electrical Properties of Hafnium Silicate Gate Dielectrics Deposited Directly on Silicon. Appl Phys Lett. 1999, 74, 2854–2856. [Google Scholar] [CrossRef]
- Lin, M.H.; Lan, C.K.; Chen, C.C.; Wu, J.Y. Electrical Properties of HfO2/La2O3 Gate Dielectrics on Ge with Ultrathin Nitride Interfacial Layer Formed by in Situ N2/H2/Ar Radical Pretreatment. Appl. Phys. Lett. 2011, 99, 182105. [Google Scholar] [CrossRef]
- Wang, S.K.; Kita, K.; Lee, C.H.; Tabata, T.; Nishimura, T.; Nagashio, K.; Toriumi, A. Desorption Kinetics of GeO from GeO2/Ge Structure. J. Appl. Phys. 2010, 105, 054104. [Google Scholar]
- Bhaisare, M.; Misra, A.; Kottantharayil, A. Aluminum Oxide Deposited by Pulsed-DC Reactive Sputtering for Crystalline Silicon Surface Passivation. IEEE J. Photovolt. 2013, 3, 930–935. [Google Scholar] [CrossRef]
- Cao, D.; Cheng, X.H.; Jia, T.T.; Xu, D.W.; Wang, Z.J.; Xia, C.; Yu, Y.H. Characterization of HfO2/La2O3 Layered Stacking Deposited on Si Substrate. J. Vac. Sci. Technol. B. Nanotechnol. Microelectron. 2013, 31, 01A113. [Google Scholar] [CrossRef]
- Lamagna, L.; Wiemer, C.; Perego, M.; Volkos, S.N.; Baldovino, S.; Tsoutsou, D.; Schamm-Chardon, S.; Coulon, P.E.; Fanciulli, M. O3-Based Atomic Layer Deposition of Hexagonal La2O3 films on Si (100) and Ge (100) Substrates. J. Appl. Phys. 2010, 108, 084108. [Google Scholar] [CrossRef]
- Martens, K.; Chui, C.O.; Brammertz, G.; Jaeger, B.D.; Kuzum, D.; Meuris, M.; Heyns, M.M.; Krishnamohan, T.; Saraswat, K.; Maes, H.E.; et al. On the Correct Extraction of Interface Trap Density of MOS Devices with High-Mobility Semiconductor Substrates. IEEE Trans. Electron Device 2008, 55, 547–556. [Google Scholar] [CrossRef]
- Suzuki, T.; Kouda, M.; Ahmet, P.; Iwai, H.; Kakushima, K.; Yasuda, T. La2O3 Gate Insulators Prepared by Atomic Layer Deposition: Optimal Growth Conditions and MgO/La2O3 Stacks for Improved Metal-Oxide-Semiconductor Characteristics. J. Vac. Sci. Technol. A 2012, 30, 051507. [Google Scholar] [CrossRef]
- Hill, W.A.; Coleman, C.C. A Single-Frequency Approximation for Interface-State Density Determination. Solid State Electron. 1980, 23, 987–993. [Google Scholar] [CrossRef]
- Stesmans, A.; Afanas’ev, V.V. Si Dangling-Bond-Type Defects at the Interface of (100) Si with Ultrathin Layers of SiOx, Al2O3, and ZrO2. Appl. Phys. Lett. 2002, 80, 1957. [Google Scholar] [CrossRef]
- Engel-Herbert, R.; Hwang, Y.; Stemmer, S. Comparison of Methods to Quantify Interface Trap Densities at Dielectric/III–V Semiconductor Interfaces. J. Appl. Phys. 2010, 108, 124101. [Google Scholar] [CrossRef]
- Bethge, O.; Zimmermann, C.; Lutzer, B.; Simsek, S.; Abermann, S.; Bertagnolli, E. ALD Grown Rare-Earth High-K Oxides on Ge: Lowering of the Interface Trap Density and EOT Scalability. ECS Trans. 2014, 64, 69–76. [Google Scholar] [CrossRef]
- Kita, K.; Suzuki, S.; Nomura, H.; Takahashi, T.; Nishimura, T.; Toriumi, A. Direct Evidence of GeO Volatilization from GeO2/Ge and Impact of Its Suppression on GeO2/Ge Metal-Insulator-Semiconductor Characteristics. Jpn. J. Appl. Phys. 2008, 47, 2349–2353. [Google Scholar] [CrossRef]
- Hauser, J.R.; Ahmed, K. Characterization of Ultra-Thin Oxides Using Electrical C-V and I-V Measurements. AIP Conf. Proc. 1998, 445, 235. [Google Scholar]
- SZE, S.M.; NG, K.K. Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; pp. 223–236. [Google Scholar]
- Spahr, H.; Bülow, T.; Nowak, C.; Hirschberg, F.; Reinker, J.; Hamwi, S.; Johannes, H.H.; Kowalsky, W. Impact of Morphological Defects on the Electrical Breakdown of Ultra-Thin Atomic Layer Deposition Processed Al2O3 Layers. Thin Solid Films 2013, 534, 172–176. [Google Scholar] [CrossRef]








| Sample | Cox (µF/cm2) | k | △VFB (mV) | Dit (eV−1cm−2) | Not (cm−2) |
|---|---|---|---|---|---|
| S1 | 1.411 | 10.69 | 685 | 9.18 × 1012 | 6.03 × 1012 |
| S2 | 1.510 | 13.43 | 504 | 9.72 × 1012 | 4.74 × 1012 |
| S3 | 1.712 | 15.97 | 269 | 3.95 × 1012 | 2.87 × 1012 |
| S4 | 1.883 | 18.46 | 152 | 2.71 × 1012 | 1.79 × 1012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Liu, H.; Wang, X.; Wang, Y.; Wang, S. Electrical Properties and Interfacial Issues of HfO2/Ge MIS Capacitors Characterized by the Thickness of La2O3 Interlayer. Nanomaterials 2019, 9, 697. https://doi.org/10.3390/nano9050697
Zhao L, Liu H, Wang X, Wang Y, Wang S. Electrical Properties and Interfacial Issues of HfO2/Ge MIS Capacitors Characterized by the Thickness of La2O3 Interlayer. Nanomaterials. 2019; 9(5):697. https://doi.org/10.3390/nano9050697
Chicago/Turabian StyleZhao, Lu, Hongxia Liu, Xing Wang, Yongte Wang, and Shulong Wang. 2019. "Electrical Properties and Interfacial Issues of HfO2/Ge MIS Capacitors Characterized by the Thickness of La2O3 Interlayer" Nanomaterials 9, no. 5: 697. https://doi.org/10.3390/nano9050697





