Preparation of a Stable Nanoscale Manganese Residue-Derived FeS@Starch-Derived Carbon Composite for the Adsorption of Safranine T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MR–FeS@SC Nanocomposite, MR–FeS, and SC
2.3. Characterization
2.4. Adsorption Experiments
2.5. Recyclability Experiments
3. Results and Discussion
3.1. Characterization of the Adsorbents
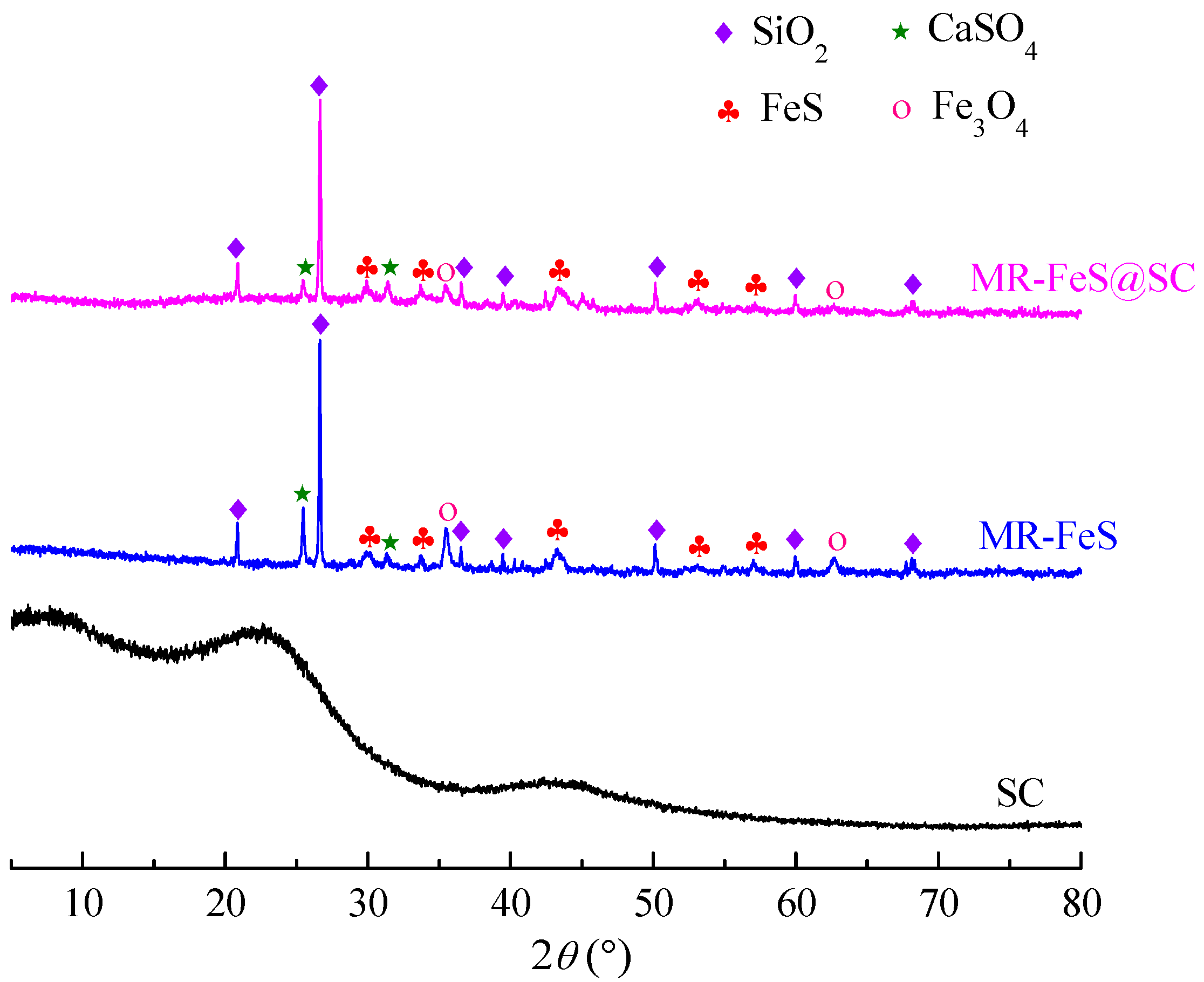
3.1.1. XRD Analysis
3.1.2. FESEM Analysis
3.1.3. Specific Surface Area and Pore Size Analysis
3.1.4. FTIR Analysis
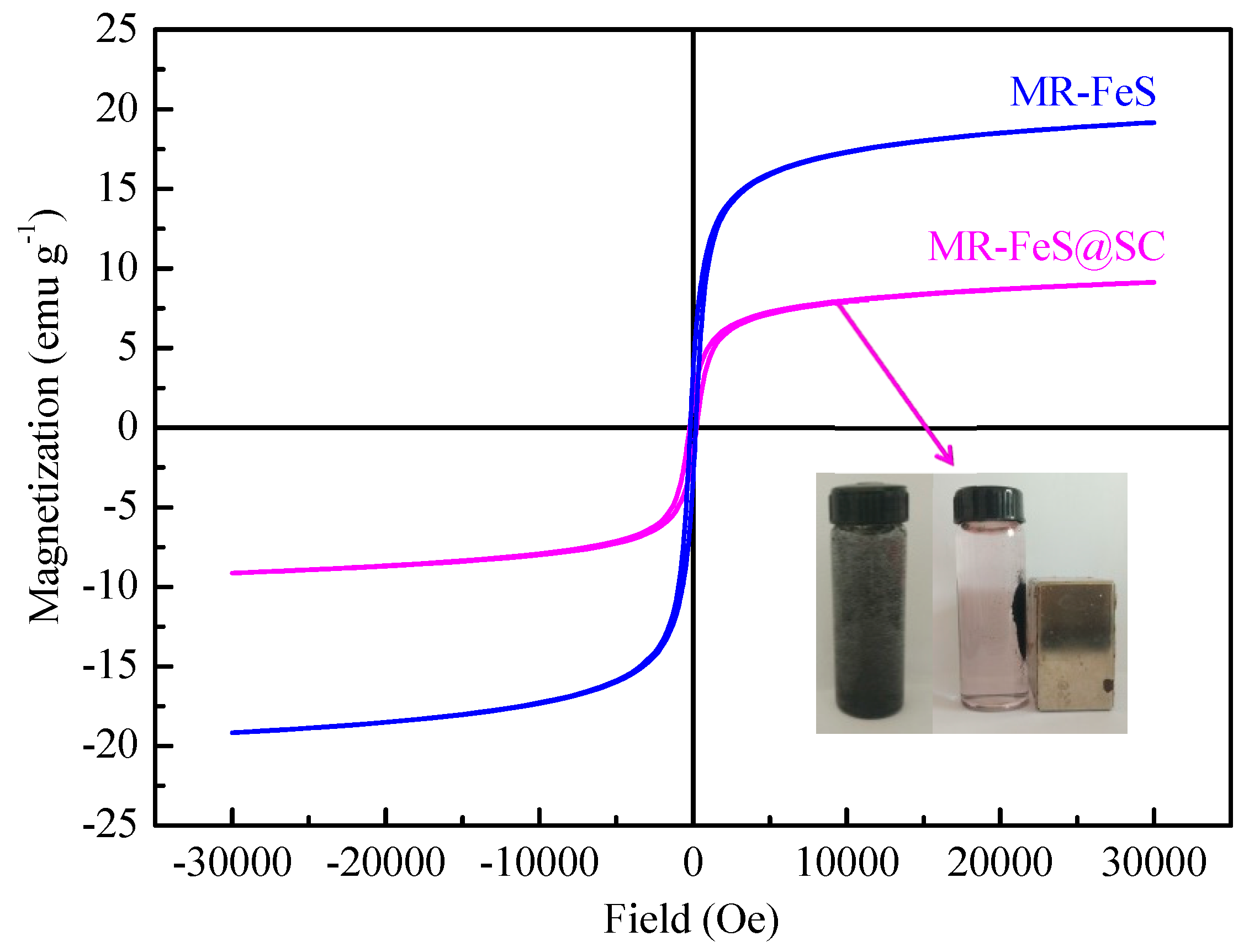
3.1.5. Magnetic Behaviors
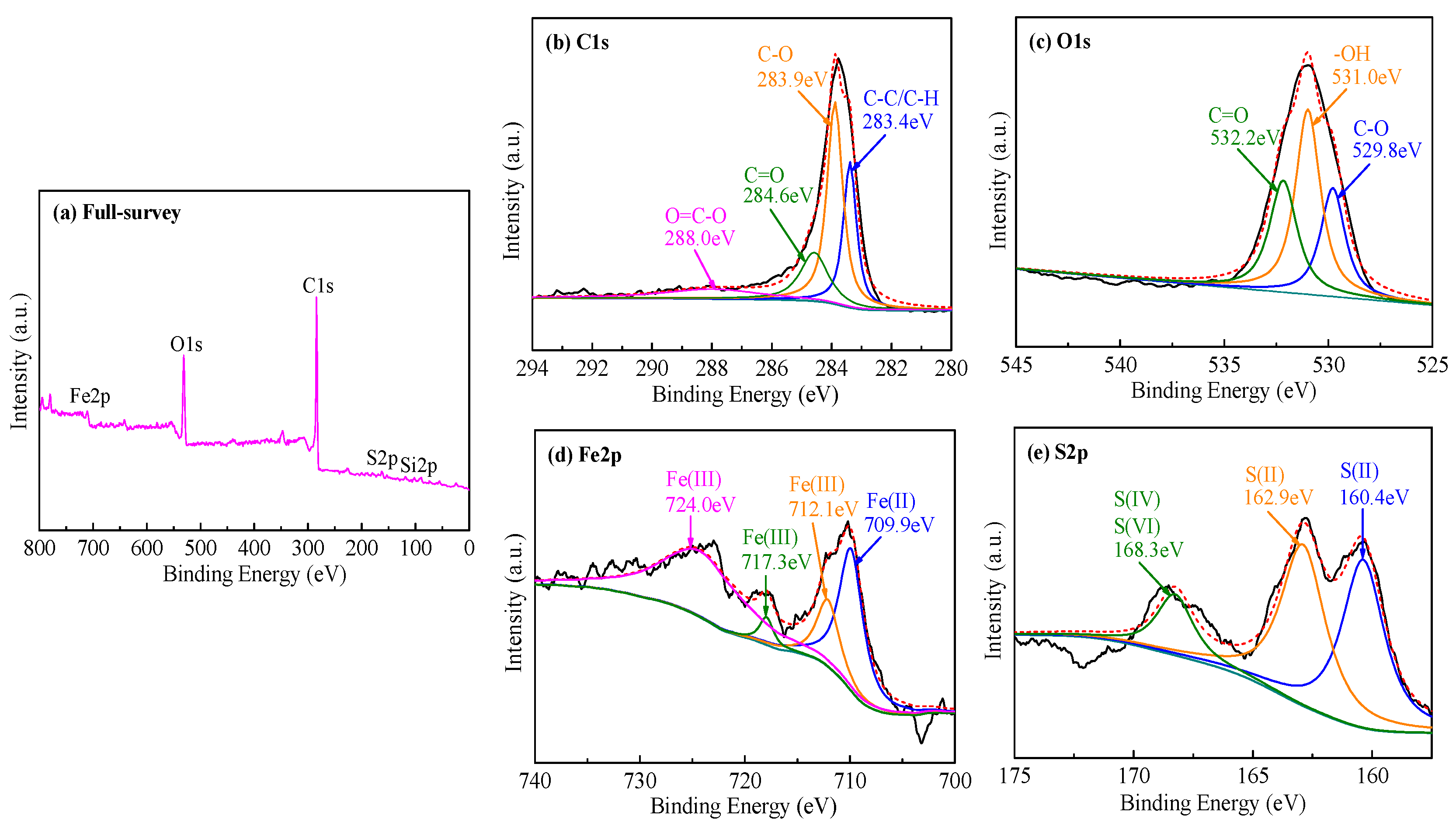
3.1.6. XPS Analysis
3.2. Adsorption of ST by the Prepared Adsorbents
3.2.1. Effect of Temperature
3.2.2. Effect of pH
3.2.3. Effect of Initial Dye Concentration
3.2.4. Effect of Contact Time
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
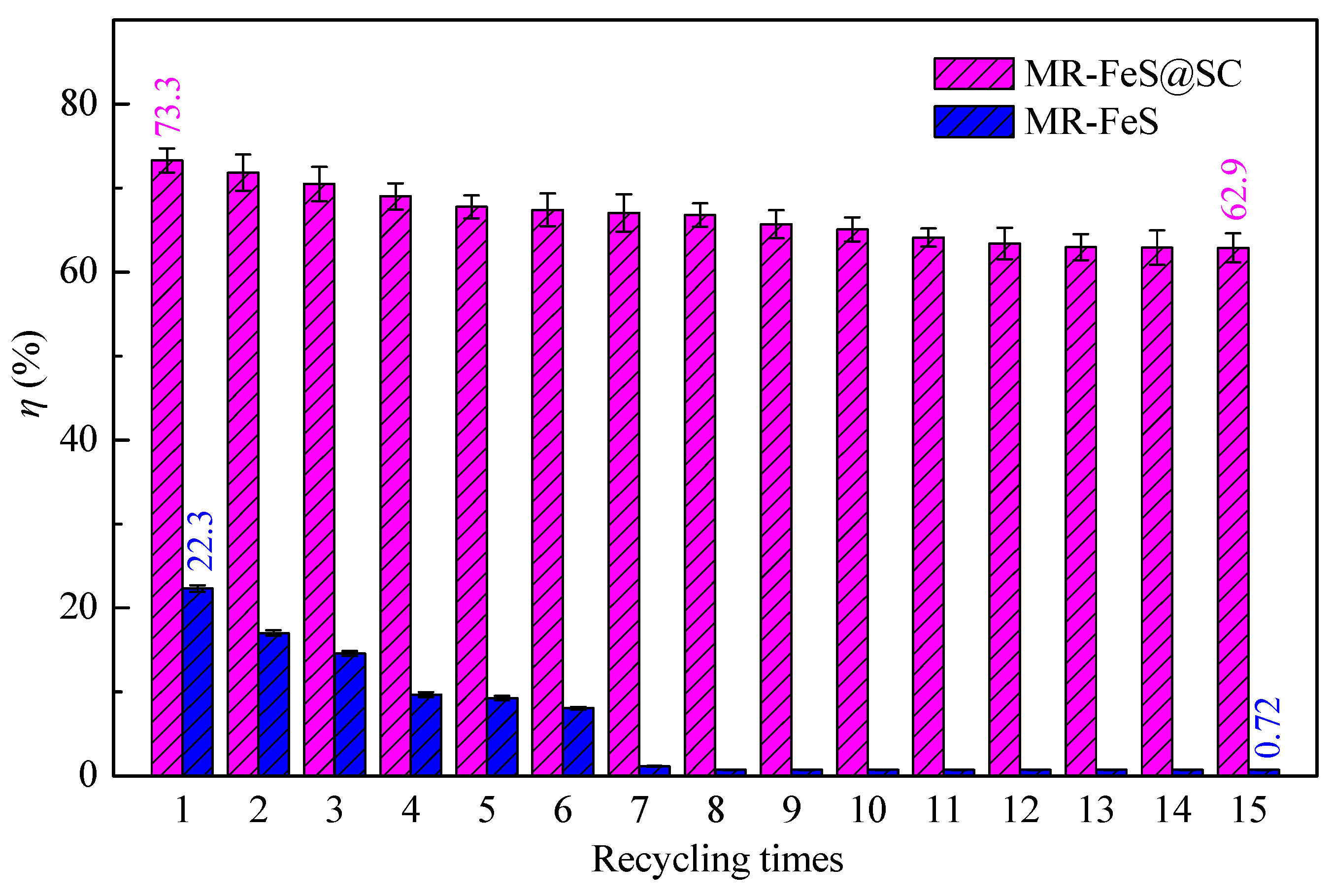
3.5. Reusability Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Liu, G.; Liu, W. Preparation of water-soluble β-cyclodextrin/poly(acrylic acid)/graphene oxide nanocomposites as new adsorbents to remove cationic dyes from aqueous solutions. Chem. Eng. J. 2014, 257, 299–308. [Google Scholar] [CrossRef]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Wang, S.; Wei, J.; Lv, S.; Guo, Z.; Jiang, F. Removal of Organic Dyes in Environmental Water onto Magnetic-Sulfonic Graphene Nanocomposite. CLEAN-Soil Air Water 2013, 41, 992–1001. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Adsorption of Reactive blue 21 onto functionalized cellulose under ultrasonic pretreatment: Kinetic and equilibrium study. J. Taiwan Inst. Chem. E 2015, 50, 229–235. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Abdelrahman, E.A. Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J. Mol. Liq. 2018, 253, 72–82. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Dai, L.; Zhu, W.; Zhu, N.; He, L.; He, M.; Tan, F.; Zhou, Q.; Hu, G. Calcium-rich biochar from crab shell: An unexpected super adsorbent for dye removal. Bioresour. Technol. 2018, 267, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, W.; Li, Y.; Wu, Y.; Chen, Y.; Xiao, W.; Zhao, L.; Zhang, J.; Li, H. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review. Chem. Eng. J. 2019, 362, 144–159. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y.; Xiong, Z.; Kaback, D.; Zhao, D. Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 2012, 23, 294007. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Gu, Y.; Xu, Y.; Zeng, G.; Hu, X.; Liu, S.; Wang, X.; Liu, S.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yang, C.; Pan, J.; Guo, Z.; Li, S. New pyrometallurgical route for separation and recovery of Fe, Zn, In, Ga and S from jarosite residues. J. Clean. Prod. 2018, 205, 781–788. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. A novel conversion process for waste residue: Synthesis of zeolite from electrolytic manganese residue and its application to the removal of heavy metals. Colloid Surface A 2015, 470, 258–267. [Google Scholar] [CrossRef]
- Pang, L.; Zou, B.; Zou, Y.; Han, X.; Cao, L.; Wang, W.; Guo, Y. A new route for the fabrication of corn starch-based porous carbon as electrochemical supercapacitor electrode material. Colloid Surf. A Physicochem. Eng. Asp. 2016, 504, 26–33. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Vaalma, C.; Bae, G.H.; Kim, G.; Passerini, S.; Kim, Y. Optimized hard carbon derived from starch for rechargeable seawater batteries. Carbon 2018, 129, 564–571. [Google Scholar] [CrossRef]
- Yao, W.; Yu, L.; Yao, P.; Wei, K.; Han, S.; Chen, P.; Xie, J. Bulk Production of Nonprecious Metal Catalysts from Cheap Starch as Precursor and Their Excellent Electrochemical Activity. ACS Sustain. Chem. Eng. 2016, 4, 3235–3244. [Google Scholar] [CrossRef]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. Enhanced adsorptive removal of Safranine T from aqueous solutions by waste sea buckthorn branch powder modified with dopamine: Kinetics, equilibrium, and thermodynamics. J. Phys. Chem. Solids 2015, 87, 23–31. [Google Scholar] [CrossRef]
- Teodoro, F.S.; Elias, M.M.C.; Ferreira, G.M.D.; Adarme, O.F.H.; Savedra, R.M.L.; Siqueira, M.F.; Da Silva, L.H.M.; Gil, L.F.; Gurgel, L.V.A. Synthesis and application of a new carboxylated cellulose derivative. Part III: Removal of auramine-O and safranin-T from mono- and bi-component spiked aqueous solutions. J. Colloid Interf. Sci. 2018, 512, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New Starch-Derived Mesoporous Carbonaceous Materials with Tunable Properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef]
- García, A.M.; Budarin, V.L.; Zhou, Y.; De Bruyn, M.; Hunt, A.J.; Lari, L.; Lazarov, V.K.; Salavagione, H.J.; Morales, E.; Ellis, G.J.; et al. Monolithic mesoporous graphitic composites as super capacitors: From Starbons to Starenes®. J. Mater. Chem. A 2018, 6, 1119–1127. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Sui, Z.; Meng, Q.; Li, J.; Zhu, J.; Cui, Y.; Han, B. High surface area porous carbons produced by steam activation of graphene aerogels. J. Mater. Chem. A 2014, 2, 9891–9898. [Google Scholar] [CrossRef]
- Tang, Z.; Pei, Z.; Wang, Z.; Li, H.; Zeng, J.; Ruan, Z.; Huang, Y.; Zhu, M.; Xue, Q.; Yu, J.; et al. Highly anisotropic, multichannel wood carbon with optimized heteroatom doping for supercapacitor and oxygen reduction reaction. Carbon 2018, 130, 532–543. [Google Scholar] [CrossRef]
- Rattanachueskul, N.; Saning, A.; Kaowphong, S.; Chumha, N.; Chuenchom, L. Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process. Bioresour. Technol. 2017, 226, 164–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, T.; Hu, H.; Huang, Z.; Huang, A.; Zhu, Y.; Feng, Z.; Yang, M. A green technology for the preparation of high fatty acid starch esters: Solid-phase synthesis of starch laurate assisted by mechanical activation with stirring ball mill as reactor. Ind. Eng. Chem. Res. 2014, 53, 2114–2120. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Qi, W.; Tong, L.; Su, R.; He, Z. Hydrolysis of cellulose by sulfonated magnetic reduced graphene oxide. Chem. Eng. J. 2015, 280, 90–98. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Sui, H.; He, L. One-Step Fabrication of Dual Responsive Lignin Coated Fe3O4 Nanoparticles for Efficient Removal of Cationic and Anionic Dyes. Nanomaterials 2018, 8, 162. [Google Scholar] [CrossRef]
- Luo, X.; Liu, S.; Zhou, J.; Zhang, L. In situ synthesis of Fe3O4/cellulose microspheres with magnetic-induced protein delivery. J. Mater. Chem. 2009, 19, 3538. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Hu, H.; Wang, W.; Huang, Z.; Chen, D.; Yang, M.; Liang, J. In Situ Synthesis of a Stable Fe3O4@Cellulose Nanocomposite for Efficient Catalytic Degradation of Methylene Blue. Nanomaterials 2019, 9, 275. [Google Scholar] [CrossRef]
- Martins, A.C.; Huang, X.; Goswami, A.; Koh, K.; Meng, Y.; Almeida, V.C.; Asefa, T. Fibrous porous carbon electrocatalysts for hydrazine oxidation by using cellulose filter paper as precursor and self-template. Carbon 2016, 102, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Briggs, D.; Seah, M.P. Practical Surface Analysis, Volume 2—Ion and Neutral Spectroscopy, 2nd ed.; Wiley: Chichester, UK, 1992. [Google Scholar]
- Mouni, L.; Belkhiri, L.; Bollinger, J.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Maatar, W.; Alila, S.; Boufi, S. Cellulose based organogel as an adsorbent for dissolved organic compounds. Ind. Crops Prod. 2013, 49, 33–42. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Wang, X.; Huang, Q.; Min, Y.; Ma, T.; Niu, J. Removal of Crystal Violet by a Novel Cellulose-Based Adsorbent: Comparison with Native Cellulose. Ind. Eng. Chem. Res. 2014, 53, 5498–5506. [Google Scholar] [CrossRef]
- Fan, S.; Huang, Z.; Zhang, Y.; Hu, H.; Liang, X.; Gong, S.; Zhou, J.; Tu, R. Magnetic chitosan-hydroxyapatite composite microspheres: Preparation, characterization, and application for the adsorption of phenolic substances. Bioresour. Technol. 2019, 274, 48–55. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, J.; Huang, A.; Guan, J.; Zhang, Q.; Huang, Z.; Hu, H.; Zhang, Y.; Yang, M.; Wu, J.; et al. A green technology for the synthesis of cellulose succinate for efficient adsorption of Cd(II) and Pb(II) ions. RSC Adv. 2016, 6, 26817–26825. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, J.; Zeng, G.; Ou, X.; Chang, Y.; Deng, C.; Zhang, J.; Liu, H.; Huang, S. Magnetic chitosan-graphene oxide composite for anti-microbial and dye removal applications. Int. J. Biol. Macromol. 2016, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin-chitosan. Colloid Surf. B 2013, 107, 76–83. [Google Scholar] [CrossRef] [PubMed]







| Element | O | Fe | Si | S | Ca | Al | Mn | Ba | K |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction (%) | 43.8 | 22.0 | 12.0 | 10.0 | 4.0 | 3.0 | 1.0 | 1.0 | 0.9 |
| Adsorbent | qe(cal) (mg g−1) | qe(exp) (mg g−1) | k2 (g mg−1 min−1) | R2 |
|---|---|---|---|---|
| SC | 10.02 | 10.01 | 0.0098 | 0.9957 |
| MR–FeS | 27.82 | 28.04 | 0.0067 | 0.9995 |
| MR–FeS@SC | 92.34 | 93.52 | 0.0016 | 0.9996 |
| Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | kL (L mg−1) | R2 | n | kF (L mg−1) | R2 | |
| SC | 32.22 | 0.054 | 0.9956 | 2.64 | 5.00 | 0.8873 |
| MR–FeS | 36.74 | 0.077 | 0.9960 | 2.96 | 7.36 | 0.8758 |
| MR–FeS@SC | 102.14 | 0.639 | 0.9918 | 4.97 | 48.06 | 0.9471 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Lin, C.; Zhang, Y.; Cai, X.; Huang, Z.; Chen, C.; Qin, Y.; Liang, J. Preparation of a Stable Nanoscale Manganese Residue-Derived FeS@Starch-Derived Carbon Composite for the Adsorption of Safranine T. Nanomaterials 2019, 9, 839. https://doi.org/10.3390/nano9060839
Hu H, Lin C, Zhang Y, Cai X, Huang Z, Chen C, Qin Y, Liang J. Preparation of a Stable Nanoscale Manganese Residue-Derived FeS@Starch-Derived Carbon Composite for the Adsorption of Safranine T. Nanomaterials. 2019; 9(6):839. https://doi.org/10.3390/nano9060839
Chicago/Turabian StyleHu, Huayu, Caiqiong Lin, Yanjuan Zhang, Xiunan Cai, Zuqiang Huang, Congjin Chen, Yuben Qin, and Jing Liang. 2019. "Preparation of a Stable Nanoscale Manganese Residue-Derived FeS@Starch-Derived Carbon Composite for the Adsorption of Safranine T" Nanomaterials 9, no. 6: 839. https://doi.org/10.3390/nano9060839





