Electrochemistry Studies of Hydrothermally Grown ZnO on 3D-Printed Graphene
Abstract
1. Introduction
2. Materials and Methods
3. Results
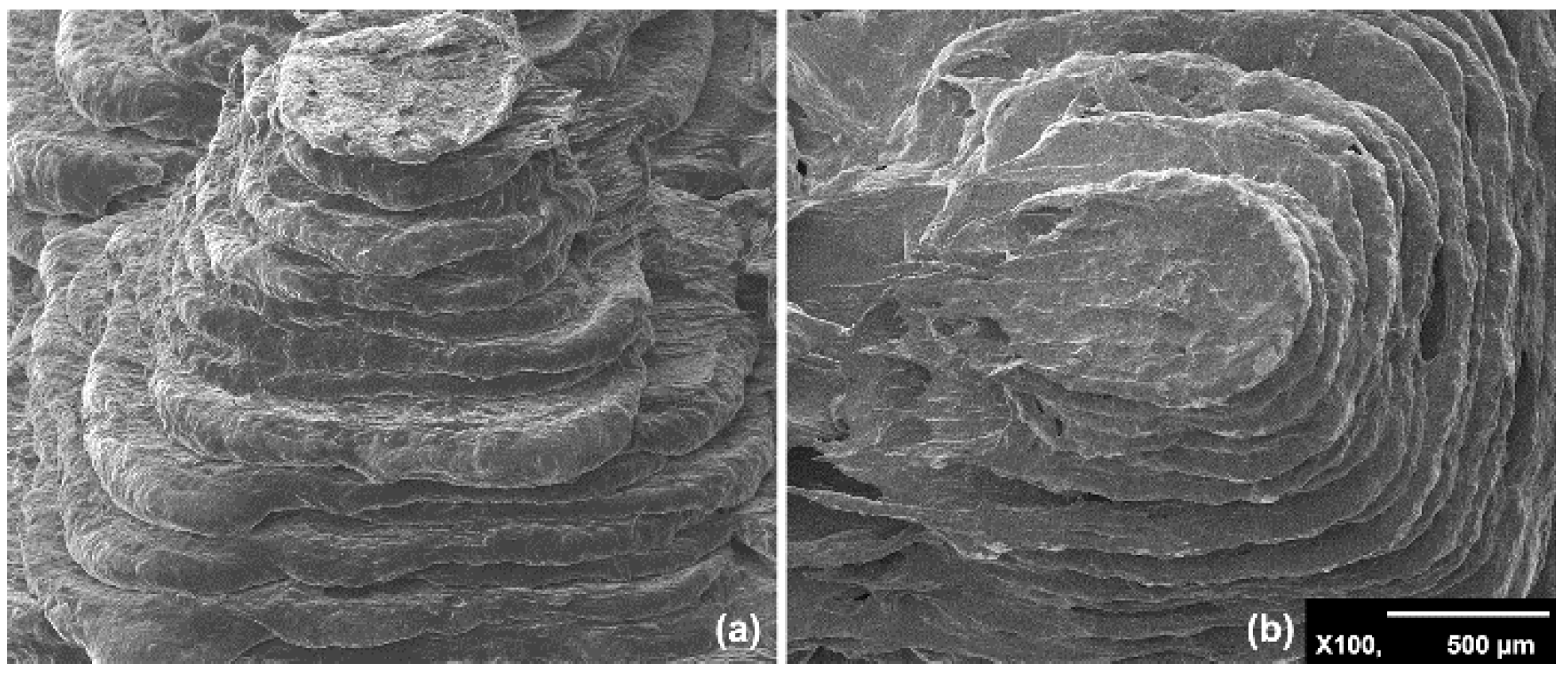
3.1. Surface Morphology
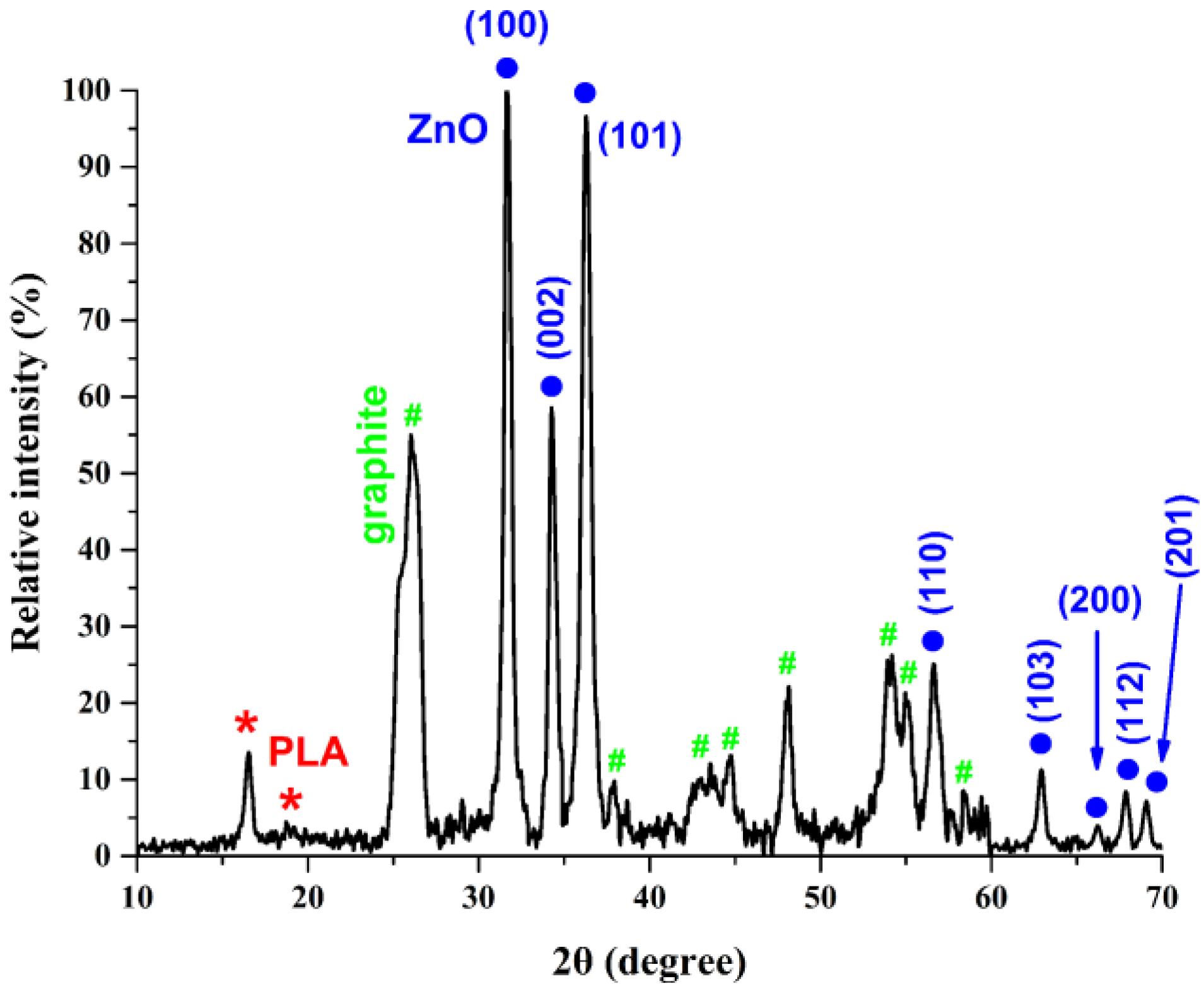
3.2. Structure
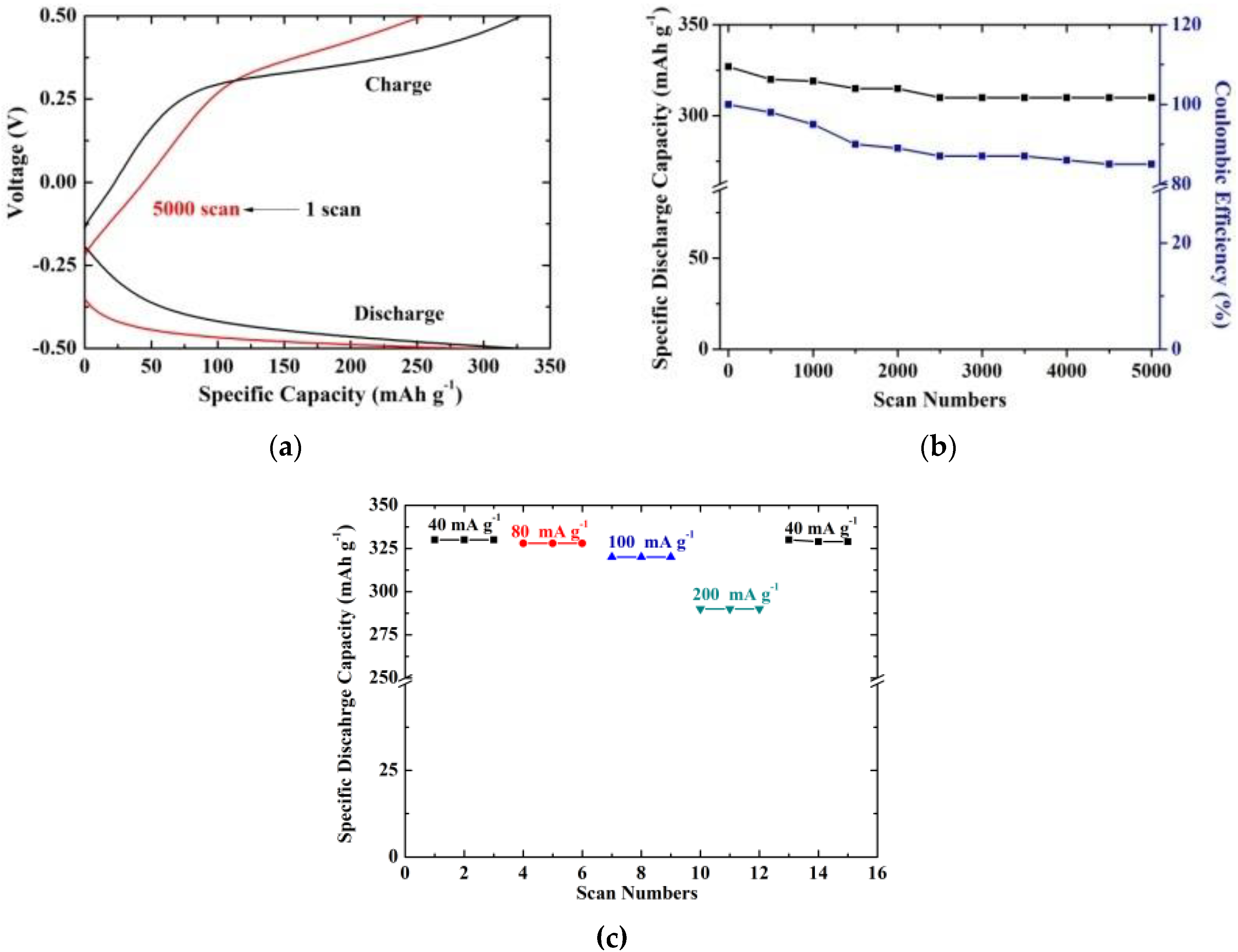
3.3. Electrochemical Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ji, L.; Lin, Z.; Alcoutalabi, M.; Zhang, X. Recent development in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuator A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Xiao, L.; Li, E.; Yi, J.; Meng, W.; Wang, S.; Deng, B.; Liu, J. Enhancing the performance of nanostructured ZnO as an anode material for lithium-ion batteries by polydopamine-derived carbon coating and confined crystallization. J. Alloy. Compd. 2018, 764, 545–554. [Google Scholar] [CrossRef]
- Su, Q.M.; Dong, Z.M.; Zhang, J. Visualizing the electrochemical reaction of ZnO nanoparticles with lithium by in situ TEM: Two reaction modes are revealed. Nanotechnology 2013, 24, 255705. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Li, H.; Zhao, Y.; Yin, F.; Wang, X. Simple fabrication of free-standing ZnO/graphene/carbon nanotube composite anode for lithium-ion batteries. Mater. Lett. 2016, 184, 235–238. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Zhang, Y.; Xie, H.; Yin, F. One-pot synthesis of radial ZnO nanoparticles deposited on graphene nanosheets as the anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2016, 11, 3179–3189. [Google Scholar] [CrossRef]
- Belliard, F.; Irvine, J.T.S. Electrochemical performance of ball-milled ZnO-SnO2, systems as anodes in lithium-ion battery. J. Power Sources 2001, 97, 219–222. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Wang, G.; Bakenov, Z. Synthesis and electrochemical investigation of highly dispersed ZnO nanoparticles as anode material for lithium-ion batteries. Ionics 2016, 22, 1387–1393. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, T.; Zhao, Y.; Liu, N. Preparation of ZnO nanorods/graphene composite anodes for high-performance lithium-ion batteries. Nanomaterials 2018, 8, 966. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Shi, Y.; Nisar, A.; Sun, H.; Shen, W.; Wei, M.; Zhu, J. Synthesis of hierarchical flower-like ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high performance Li-ion battery anodes. J. Mater. Chem. 2011, 21, 7723–7729. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Liu, Y.; Zheng, P.; Yuan, X.; Guo, S. Al doped-ZnO nanoparticles implanted in reduced graphene oxide with improved electrochemical properties for lithium ion batteries. Mater. Lett. 2016, 165, 165–168. [Google Scholar] [CrossRef]
- Park, K.; Xia, F.; Kim, S.; Song, T.; Paik, U.; Park, W. Facile synthesis of ultrathin ZnO nanotubes with well-organized hexagonal nanowalls and sealed layouts: Applications for lithium ion battery anodes. J. Phys. Chem. C 2013, 117, 1037–1043. [Google Scholar] [CrossRef]
- Xiao, L.; Mei, D.; Cao, M.; Qu, D.; Deng, B. Effects of structural patterns and degree of crystallinity on the performance of nanostructured ZnO as anode material for lithium-ion batteries. J. Alloy Compd. 2015, 627, 455–462. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Wu, Z.S.; Zhou, G.; Yin, L.C.; Ren, W.; Li, F.; Cheng, H.M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, C.Y.; Chen, Y.F.; Lin, J.S. Synthesis of ZnO@Graphene composites as anode materials for lithium ion batteries. Electrochim. Acta 2013, 111, 359–365. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Zhang, C.; Wang, G.; Zhao, Y.; Yin, F.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Shen, X.; Mu, D.; Chen, S.; Wu, B.; Wu, F. Enhanced electrochemical performance of ZnO-loaded/porous carbon composite as anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3118–3125. [Google Scholar] [CrossRef]
- Vernardou, D.; Vasilopoulos, K.C.; Kenanakis, G. 3D printed graphene-based electrodes with high electrochemical performance. Appl. Phys. A 2017, 123, 623. [Google Scholar] [CrossRef]
- Kenanakis, G.; Androulidaki, M.; Vernardou, D.; Katsarakis, N.; Koudoumas, E. Photoluminescence study of ZnO structures grown by aqueous chemical growth. Thin Solid Films 2011, 520, 1353–1357. [Google Scholar] [CrossRef]
- Vernardou, D.; Kenanakis, G.; Vlachou, K.; Koudoumas, E.; Kiriakidis, G.; Vairis, A.; Katsarakis, N. Influence of solution concentration and temperature on the aqueous chemical growth of zinc oxide growth. Phys. Status Sol. 2008, 5, 3348–3352. [Google Scholar] [CrossRef]
- Thongam, D.D.; Gupta, J.; Sahu, N.K.; Bahadur, D. Investigating the role of different reducing agents, molar ratios, and synthesis medium over the formation of ZnO nanostructures and their photo-catalytic activity. J. Mater. Sci. 2018, 53, 1110–1122. [Google Scholar] [CrossRef]
- Kenanakis, G.; Vernardou, D.; Koudoumas, E.; Katsarakis, N. Growth of c-axis oriented ZnO nanowires from aqueous solution: The decisive role of seed layer for controlling the wires’ diameter. J. Cryst. Growth 2009, 311, 4799–4804. [Google Scholar] [CrossRef]
- Vernardou, D.; Apostolopoulou, M.; Louloudakis, D.; Katsarakis, N.; Koudoumas, E. Hydrothermally grown β-V2O5 electrode at 95 °C. J. Colloid. Interf. Sci. 2014, 424, 1–6. [Google Scholar] [CrossRef]
- Teixeira, E.M.; de Campos, A.; Marconcini, J.M.; Bondancia, T.J.; Wood, D.; Klamczynski, A.; Mattoso, L.H.C.; Glenn, G.M. Starch/fiber/poly(lactic acid) foam and compressed foam composites. RSC Adv. 2014, 4, 6616–6623. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Yu, L.; Li, Z.; Sun, S. Preparation of high-quality biocompatible carbon dots by extraction, with new thoughts on the luminescence mechanism. Nanotechnology 2013, 24, 225601–225608. [Google Scholar] [CrossRef]
- Howe, J.Y.; Rawn, C.J.; Jones, L.E.; Ow, H. Improved crystallographic data for graphite. Powder Diffr. 2003, 18, 150–154. [Google Scholar] [CrossRef]
- Vernardou, D.; Kenanakis, G.; Couris, S.; Koudoumas, E.; Kymakis, E.; Katsarakis, N. pH effect on the morphology of ZnO nanostructures grown with aqueous chemical growth. Thin Solid Films 2007, 515, 8764–8767. [Google Scholar] [CrossRef]
- Park, S.H.; Bak, S.M.; Kim, K.H.; Jegal, J.P.; Lee, S.I.; Lee, J.; Kim, K.B. Solid-state microwave irradiation synthesis of high quality graphene nanosheets under hydrogen containing atmosphere. J. Mater. Chem. 2011, 21, 680–686. [Google Scholar] [CrossRef]
- Shen, J.; Li, T.; Long, Y.; Shi, M.; Li, N.; Ye, M. One-step solid state preparation of reduced graphene oxide. Carbon 2012, 50, 2134–2140. [Google Scholar] [CrossRef]
- Zhaochun, Z.; Baibiao, H.; Yongqin, Y.; Deliang, C. Electrical properties and Raman spectra of undoped and Al-doped ZnO thin films by metalorganic vapor phase epitaxy. Mater. Sci. Eng. B 2001, 86, 109–112. [Google Scholar] [CrossRef]
- Cusco, R.; Alarcon-Liado, E.; Ibanez, J.; Artus, L.; Jimenez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Cerqueira, M.F.; Viseu, T.; Ayres de Campos, J.; Rolo, A.G.; de Lacerda-Aroso, T.; Oliveira, F.; Bogdanovic-Radovic, I.; Alves, E.; Vasilevskiy, M.I. Raman study of insulating and conductive ZnO:(Al, Mn) thin films. Phys. Status Solidi 2015, 212, 2345–2354. [Google Scholar] [CrossRef]
- Vano-Herrera, K.; Misiun, A.; Vogt, C. Preparation and characterization of poly(lactic acid)/poly(methyl methacrylate) blend tablets for application in quantitative analysis by micro Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 273–279. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Huang, X.H.; Xia, X.H.; Yuan, Y.F.; Zhou, F. Porous ZnO nanosheets grown on copper substrates as anode for lithium ion batteries. Electrochim. Acta 2011, 56, 4960–4965. [Google Scholar] [CrossRef]
- Huang, X.H.; Wu, J.B.; Lin, Y.; Guo, R.Q. ZnO microrod arrays grown on copper substrates as anode materials for lithium ion batteries. Int. J. Electrochem. Sci. 2012, 7, 6611–6621. [Google Scholar]
- Wu, Z.; Qin, L.; Pan, Q. Fabrication and electrochemical behaviour of flower-like ZnO-CoO-C nanowall arrays as anodes for lithium-ion batteries. J. Alloy Compd. 2011, 509, 9207–9213. [Google Scholar] [CrossRef]
- Moon, Y.W.; Choi, I.J.; Koh, Y.H.; Kim, H.E. Porous alumina ceramic scaffolds with biomimetic macro/micro-porous structure using three-dimensional (3-D) ceramic/camphene-based extrusion. Ceram. Int. 2015, 41, 12371–12377. [Google Scholar] [CrossRef]
- Lee, J.H.; Hon, M.H.; Chung, Y.W. The effect of TiO2 coating on the electrochemical performance of ZnO nanorod as the anode material for lithium-ion battery. Appl. Phys. A Mater. Sci. Process. 2011, 102, 545–550. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Liu, H.; Liu, Z.Q. SiO2@C hollow sphere anodes for lithium-ion batteries. J. Mater. Sci. Technol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ding, J.J.; Hu, Y.; Ji, X.; Chi, Q.; Zhu, Z.; Huang, X. Carbon/ZnO nanorod array electrode with significantly improved lithium storage capability. J. Phys. Chem. C 2009, 113, 5336–5339. [Google Scholar] [CrossRef]


| Anodes | Specific Discharge Capacity | Scan Number | References |
|---|---|---|---|
| ZnO-CoO-C | 438 mAh g−1 | 50 (0.0–2.5 V) | [40] |
| ZnO/graphene | 250 mAh g−1 | 100 (0.005–3.0 V) | [41] |
| ZnO-Loaded/Porous carbon composite | 512.7 mAh g−1 | 10 (0.1–3.0 V) | [20] |
| ZnO nanorod | 358 mAh g−1 | 30 (0.3–3.0 V) | [42] |
| 3D-printed graphene | 265 mAh g−1 | 1000(−1.0–0.5 V) | [21] |
| ZnO/3D-printed graphene pyramids | 306 mAh g−1 | 5000 (−1.0–0.5 V) | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernardou, D.; Kenanakis, G. Electrochemistry Studies of Hydrothermally Grown ZnO on 3D-Printed Graphene. Nanomaterials 2019, 9, 1056. https://doi.org/10.3390/nano9071056
Vernardou D, Kenanakis G. Electrochemistry Studies of Hydrothermally Grown ZnO on 3D-Printed Graphene. Nanomaterials. 2019; 9(7):1056. https://doi.org/10.3390/nano9071056
Chicago/Turabian StyleVernardou, Dimitra, and George Kenanakis. 2019. "Electrochemistry Studies of Hydrothermally Grown ZnO on 3D-Printed Graphene" Nanomaterials 9, no. 7: 1056. https://doi.org/10.3390/nano9071056
APA StyleVernardou, D., & Kenanakis, G. (2019). Electrochemistry Studies of Hydrothermally Grown ZnO on 3D-Printed Graphene. Nanomaterials, 9(7), 1056. https://doi.org/10.3390/nano9071056






