Flexible HIV-1 Biosensor Based on the Au/MoS2 Nanoparticles/Au Nanolayer on the PET Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MoS2 NPs Synthesis
2.3. Fabrication of the Au/MoS2/Au Nanolayer on the PET Substrate, and Immobilization of the gp120 Antibody
2.4. Electrochemical Properties of the Au/MoS2/Au Nanolayer on the PET Substrate
2.5. Flexibility Test of Fabricated Biosensor
3. Results and Discussion
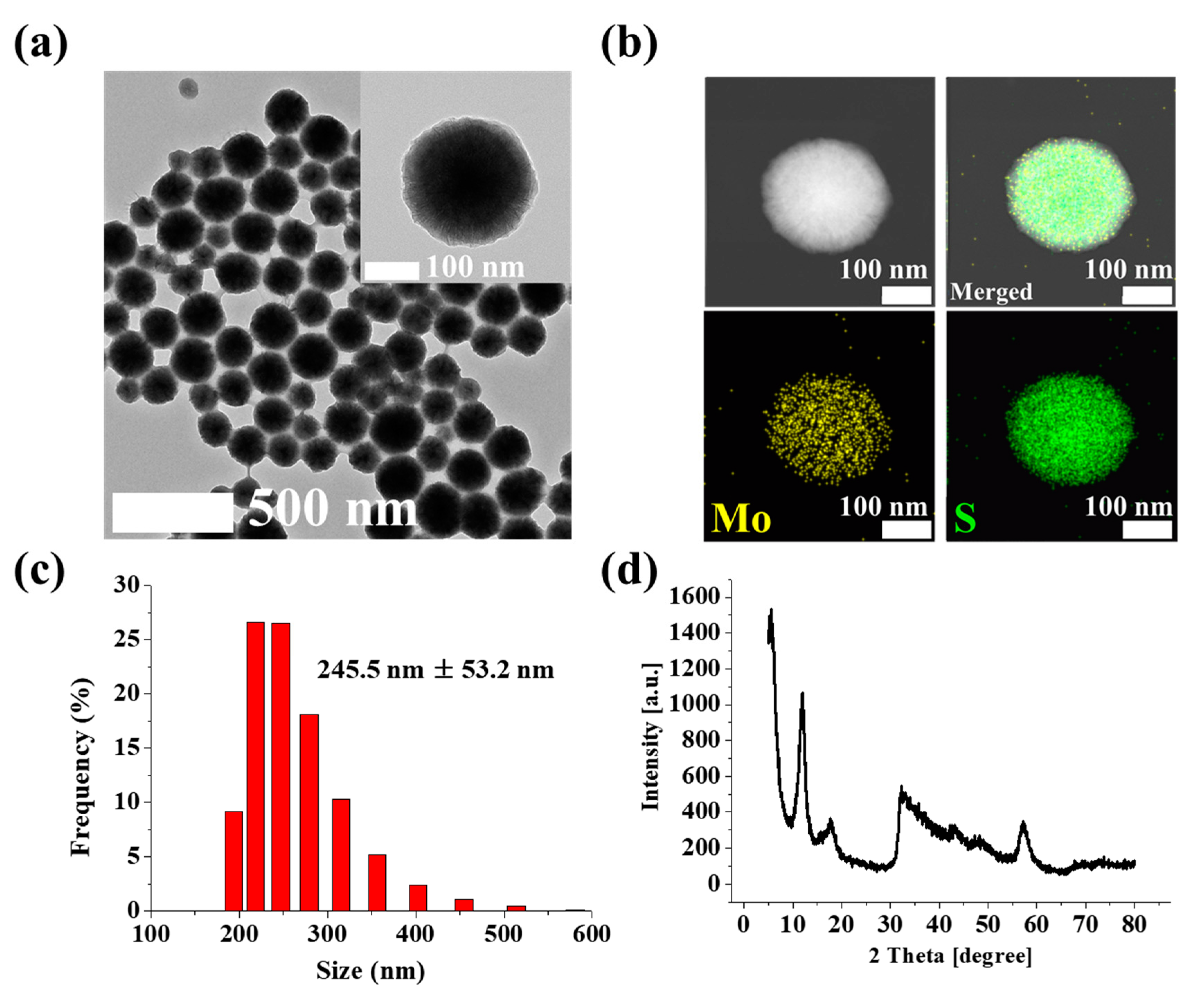
3.1. Confirmation of MoS2 NPs Synthesis
3.2. Verification of the Au/MoS2/Au Nanolayer on the PET Substrate
3.3. Investigation of the Electrochemical Properties of the Fabricated Biosensor
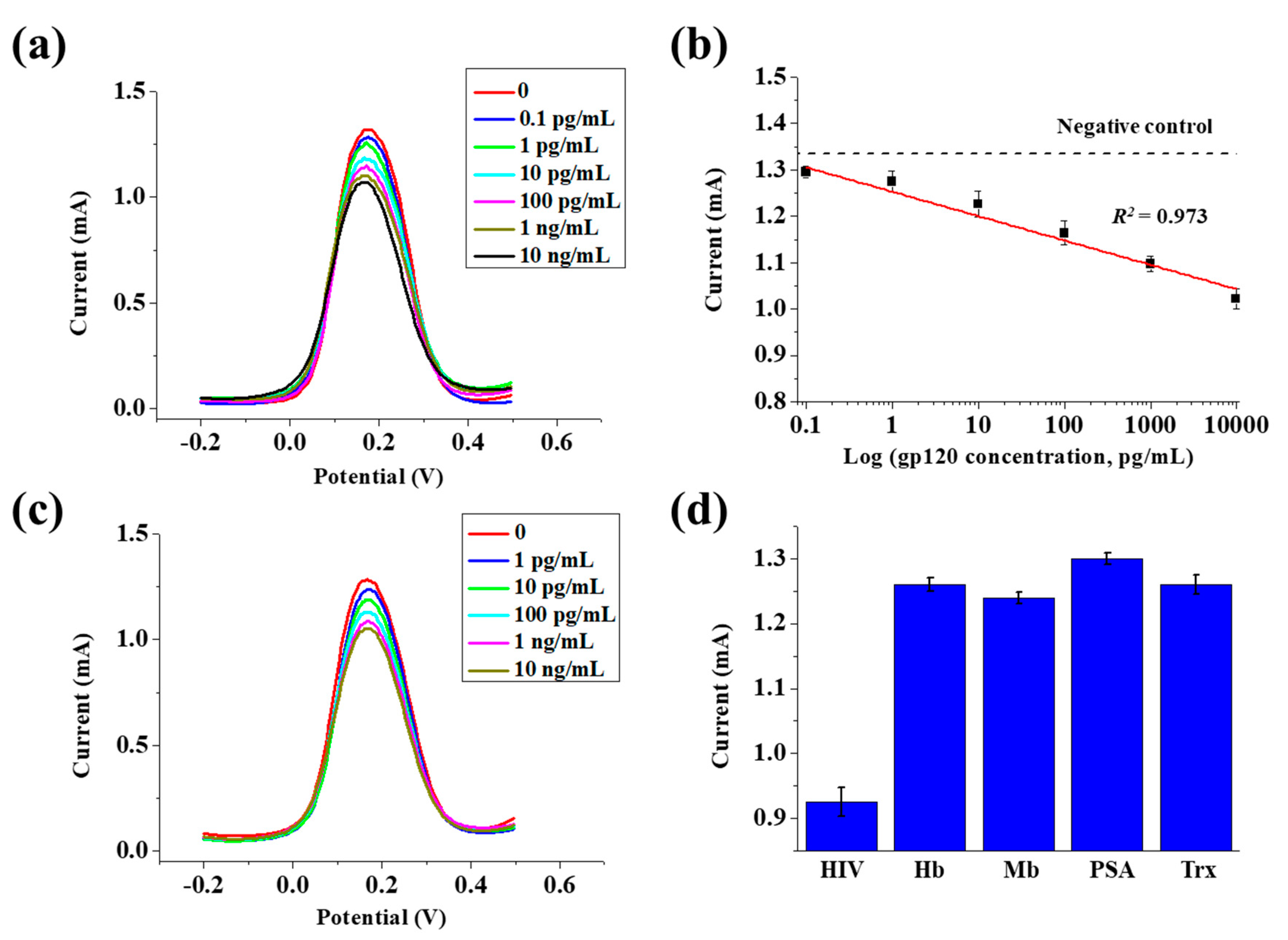
3.4. Detection of the gp120 Antigen and Selectivity
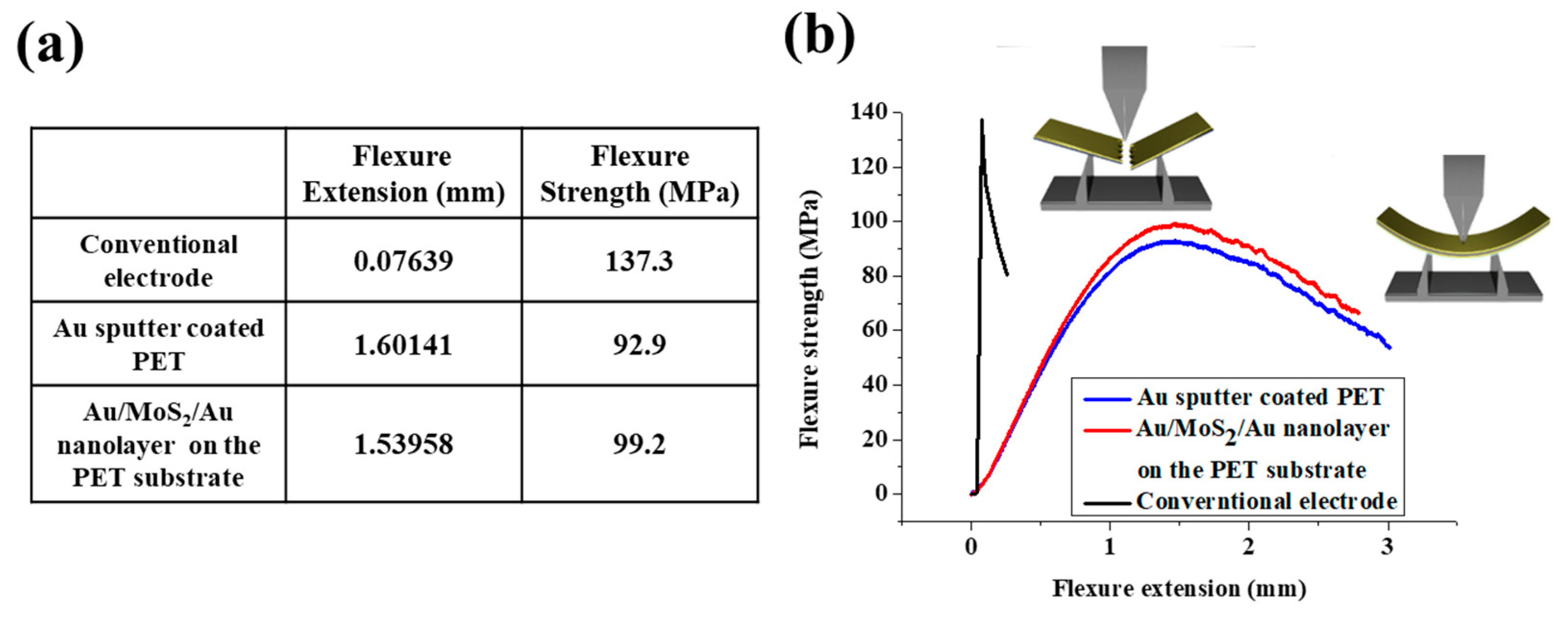
3.5. Investigation of the Flexibility of the Au/MoS2/Au Nanolayer on the PET Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Luo, C.; Jia, J.; Sun, Y.; Fu, Q.; Pan, C. A Wrinkled Ag/CNTs-PDMS Composite Film for a High-Performance Flexible Sensor and Its Applications in Human-Body Single Monitoring. Nanomaterials 2019, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tao, L.; Hao, Y.; Liu, Z.; Chou, H.; Kholmanov, I.; Chen, S.; Tan, C.; Jayant, N.; Yu, Q.; et al. Direct delamination of graphene for high-performance plastic electronics. Small 2014, 10, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Yoon, J.; Lee, T.; Cho, H.-Y.; Lee, J.-Y.; Choi, J.-W. H2O2 biosensor consisted of hemoglobin-DNA conjugate on nanoporous gold thin film electrode with electrochemical signal enhancement. Nano Converg. 2019, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Kurdekar, A.D.; Avinash Chunduri, L.A.; Manohar, C.S.; Haleyurgirisetty, M.K.; Hewlett, I.K.; Venkataramaniah, K. Streptavidin-conjugated gold nanoclusters as ultrasensitive fluorescent sensors for early diagnosis of HIV infection. Sci. Adv. 2018, 4, eaar6280. [Google Scholar] [CrossRef]
- Hsu, M.-S.; Chen, Y.-L.; Lee, C.-Y.; Chiu, H.-T. Gold Nanostructures on Flexible Substrates as Electrochemical Dopamine Sensors. ACS Appl. Mater. Interfaces 2012, 4, 5570–5575. [Google Scholar] [CrossRef]
- Loghin, F.; Rivadeneyra, A.; Becherer, M.; Lugli, P.; Bobinger, M. A Facile and Efficient Protocol for Preparing Residual-Free Single-Walled Carbon Nanotube Films for Stable Sensing Applications. Nanomaterials 2019, 9, 471. [Google Scholar] [CrossRef]
- Lin, S.; Feng, W.; Miao, X.; Zhang, X.; Chen, S.; Chen, Y.; Wang, W.; Zhang, Y. A flexible and highly sensitive nonenzymatic glucose sensor based on DVD-laser scribed graphene substrate. Biosens. Bioelectron. 2018, 110, 89–96. [Google Scholar] [CrossRef]
- El-Said, W.A.; Yoon, J.; Choi, J.-W. Nanostructured surfaces for analysis of anticancer drug and cell diagnosis based on electrochemical and SERS tools. Nano Converg. 2018, 5, 11. [Google Scholar] [CrossRef]
- Mohapatra, J.; Ananthoju, B.; Nair, V.; Mitra, A.; Bahadur, D.; Medhekar, N.V.; Aslam, M. Enzymatic and non-enzymatic electrochemical glucose sensor based on carbon nano-onions. Appl. Surf. Sci. 2018, 442, 332–341. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Li, P.; Liu, H.; Xu, H. A high-sensitive and fast-fabricated glucose biosensor based on Prussian blue/topological insulator Bi2Se3 hybrid film. Biosens. Bioelectron. 2012, 38, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mazánek, V.; Mayorga-Martinez, C.C.; Bouša, D.; Sofer, Z.; Pumera, M. WSe2 nanoparticles with enhanced hydrogen evolution reaction prepared by bipolar electrochemistry: Application in competitive magneto-immunoassay. Nanoscale 2018, 10, 23149–23156. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.-W.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zou, M.; Zhao, H.; Yuan, C.; Xu, Y.; Zhang, C.; Wang, L.; Fan, C.; Wang, L. Shape-controlled gold nanoparticles supported on MoS2 nanosheets: Synergistic effect of thionine and MoS2 and their application for electrochemical label-free immunosensing. Nanoscale 2015, 7, 19129–19135. [Google Scholar] [CrossRef]
- Kim, S.J.; Mondal, S.; Min, B.K.; Choi, C.-G. Highly Sensitive and Flexible Strain–Pressure Sensors with Cracked Paddy-Shaped MoS2 /Graphene Foam/Ecoflex Hybrid Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 36377–36384. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, U.; Phan, D.-T.; Uddin, A.S.M.I.; Chung, G.-S. Highly flexible room temperature NO2 sensor based on MWCNTs-WO3 nanoparticles hybrid on a PET substrate. Sens. Actuators B Chem. 2015, 221, 760–768. [Google Scholar] [CrossRef]
- Königer, T.; Münstedt, H. Coatings of indium tin oxide nanoparticles on various flexible polymer substrates: Influence of surface topography and oscillatory bending on electrical properties. J. Soc. Inf. Disp. 2008, 16, 559. [Google Scholar] [CrossRef]
- Saxena, S.K.; Tiwari, S.; Nair, M.P.N. A Global Perspective on HIV/AIDS. Science 2012, 337, 798. [Google Scholar] [CrossRef]
- Embretson, J.; Zupancic, M.; Ribas, J.L.; Burke, A.; Racz, P.; Tenner-Racz, K.; Haase, A.T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 1993, 362, 359–362. [Google Scholar] [CrossRef]
- Pilcher, C.D.; Tien, H.C.; Eron, J.J.; Vernazza, P.L.; Leu, S.-Y.; Stewart, P.W.; Goh, L.-E.; Cohen, M.S. Brief but Efficient: Acute HIV Infection and the Sexual Transmission of HIV. J. Infect. Dis. 2004, 189, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Moon, S.; Kuritzkes, D.R.; Demirci, U. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens. Bioelectron. 2009, 25, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Liu, J.; Bess, J.; Chertova, E.; Lifson, J.D.; Grisé, H.; Ofek, G.A.; Taylor, K.A.; Roux, K.H. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 2006, 441, 847–852. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef] [PubMed]
- McFall, S.M.; Wagner, R.L.; Jangam, S.R.; Yamada, D.H.; Hardie, D.; Kelso, D.M. A simple and rapid DNA extraction method from whole blood for highly sensitive detection and quantitation of HIV-1 proviral DNA by real-time PCR. J. Virol. Methods 2015, 214, 37–42. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lai, Z.-L.; Wang, G.-J.; Wu, C.-Y. Polymerase chain reaction-free detection of hepatitis B virus DNA using a nanostructured impedance biosensor. Biosens. Bioelectron. 2016, 77, 603–608. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, C.; Wang, M.; Wang, L.-S. Sensitive electrochemical detection of gp120 based on the combination of NBD-556 and gp120. Talanta 2019, 196, 486–492. [Google Scholar] [CrossRef]
- Lines, J.A.; Yu, Z.; Dedkova, L.M.; Chen, S. Design and expression of a short peptide as an HIV detection probe. Biochem. Biophys. Res. Commun. 2014, 443, 308–312. [Google Scholar] [CrossRef]
- John, S.V.; Rotherham, L.S.; Khati, M.; Mamba, B.B.; Arotiba, O.A. Towards HIV Detection: Novel Poly(propylene imine) Dendrimer-Streptavidin Platform for Electrochemical DNA and gp120 Aptamer Biosensors. Int. J. Electrochem. Sci. 2014, 9, 13. [Google Scholar]
- Lee, J.-H.; Oh, B.-K.; Choi, J.-W. Electrochemical sensor based on direct electron transfer of HIV-1 virus at Au nanoparticle modified ITO electrode. Biosens. Bioelectron. 2013, 49, 531–535. [Google Scholar] [CrossRef]



| Electrode | Technique | Target | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|---|
| GCE/CNF-Bi/MIP/NBD–556@ gp120 | DPV | gp120 | 0.3 pg/mL | 0.002–200 ng/mL | [27] |
| FP-50 fusion peptide | Dot blot | gp120 | - | 0–100 pg/mL | [28] |
| Poly(propylene imine) Dendrimer-Streptavidin Platform | SWV | gp120 | 4.12 pg/ml | 12.04 ng/mL–0.19 μg/mL | [29] |
| Au nonodot/ITO | CV | HIV-1 VLP(gp120) | - | 0.6–375 pg/mL | [30] |
| Ab/Cys/Au/MoS2/Au nanolayer on the PET substrate | SWV | gp120 | 0.066 pg/mL | 0.1 pg/mL–10 ng/mL | This research |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.; Yoon, J.; Yi, C.; Lee, T.; Choi, J.-W. Flexible HIV-1 Biosensor Based on the Au/MoS2 Nanoparticles/Au Nanolayer on the PET Substrate. Nanomaterials 2019, 9, 1076. https://doi.org/10.3390/nano9081076
Shin M, Yoon J, Yi C, Lee T, Choi J-W. Flexible HIV-1 Biosensor Based on the Au/MoS2 Nanoparticles/Au Nanolayer on the PET Substrate. Nanomaterials. 2019; 9(8):1076. https://doi.org/10.3390/nano9081076
Chicago/Turabian StyleShin, Minkyu, Jinho Yoon, Chanyong Yi, Taek Lee, and Jeong-Woo Choi. 2019. "Flexible HIV-1 Biosensor Based on the Au/MoS2 Nanoparticles/Au Nanolayer on the PET Substrate" Nanomaterials 9, no. 8: 1076. https://doi.org/10.3390/nano9081076
APA StyleShin, M., Yoon, J., Yi, C., Lee, T., & Choi, J.-W. (2019). Flexible HIV-1 Biosensor Based on the Au/MoS2 Nanoparticles/Au Nanolayer on the PET Substrate. Nanomaterials, 9(8), 1076. https://doi.org/10.3390/nano9081076






