Enhancement in Photoelectrochemical Performance of Optimized Amorphous SnS2 Thin Film Fabricated through Atomic Layer Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tin Disulfide Film Synthesis and Characterization
2.2. Optical Properties of the Amorphous SnS2 Films
2.3. Photoelectrochemical Performances of the Amorphous SnS2 Films
3. Results and Discussion
3.1. Tin Disulfide Film Deposition
3.1.1. Atomic layer deposition Model
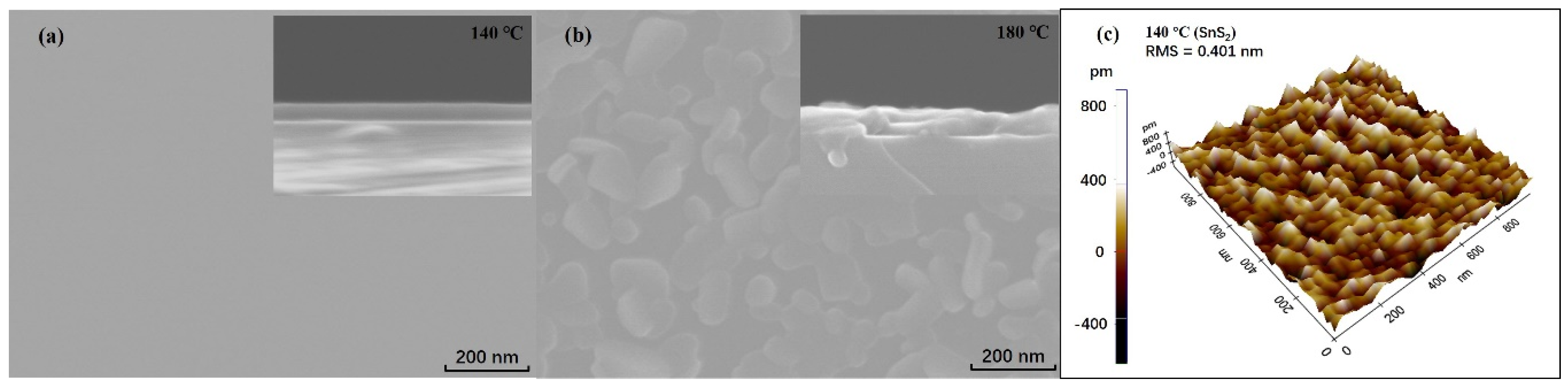
3.1.2. Surface Morphology and Roughness
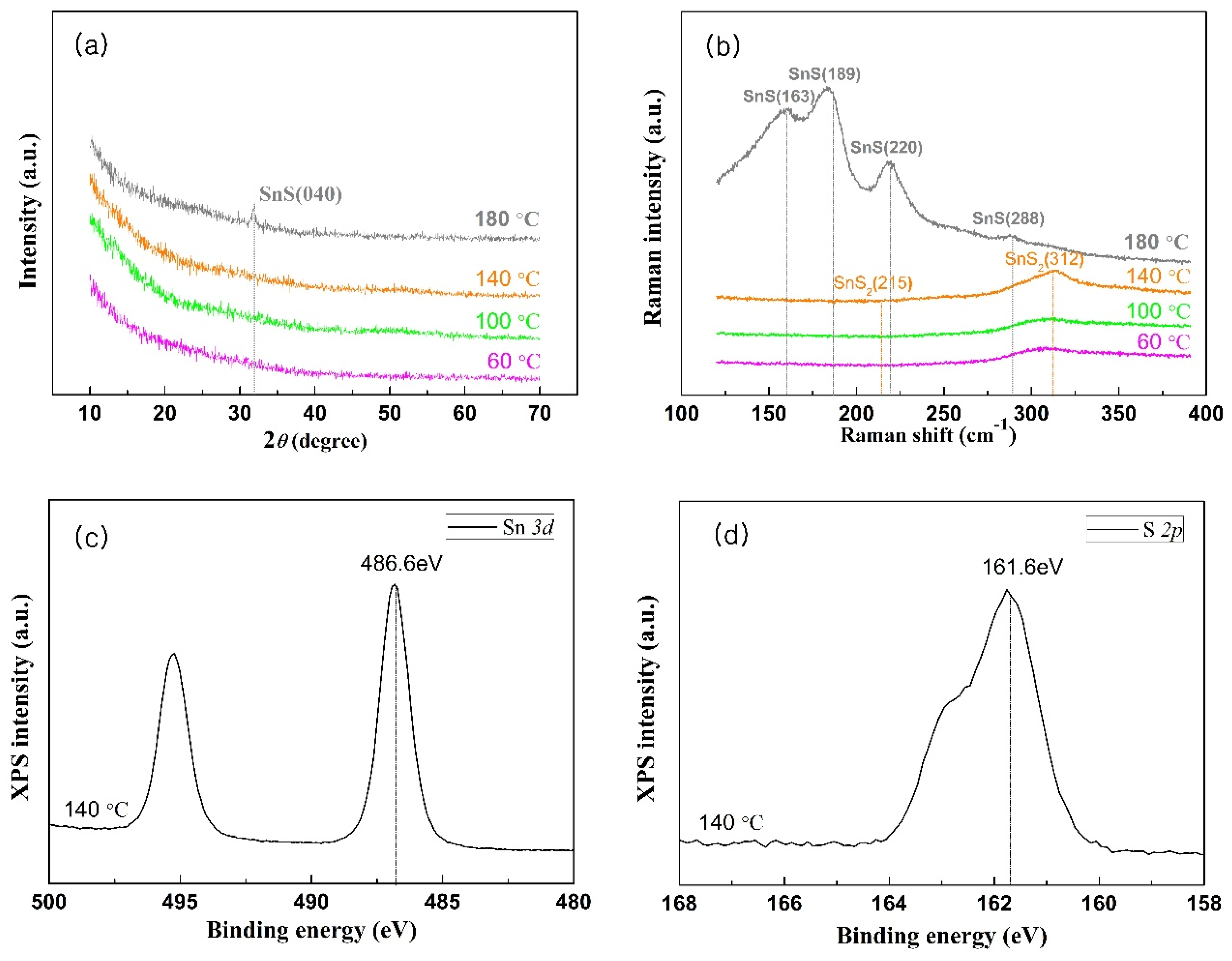
3.1.3. Crystal Structure and Phase Analysis
3.2. Optimization of the Optical Properties
3.3. Photoelectrochemical Performances and Electrochemical Impedance Spectroscopy
3.3.1. Photoelectrochemical Performances
3.3.2. Electrochemical Impedance Spectroscopy
3.3.3. Photocurrent Response Stabilities
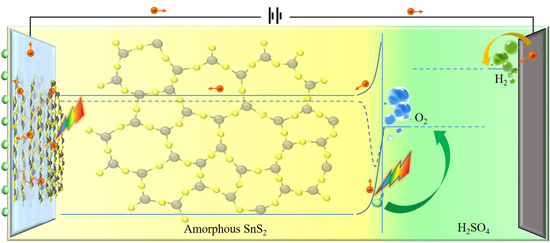
3.4. Mechanisms of Photoelectron Separation and Photoelectrochemical Water Splitting
3.4.1. Mechanism of Photoelectron Separation
3.4.2. Mechanism of Photoelectrochemical Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kemppainen, E.; Halme, J.; Lund, P. Physical Modeling of Photoelectrochemical Hydrogen Production Devices. J. Phys. Chem. C 2015, 119, 21747–21766. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Vidu, R.; Stroeve, P. Solar Energy Storage Methods. Ind. Eng. Chem. Res. 2011, 50, 8954–8964. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Zhang, Synthesis of Ultrathin CdS Nanosheets as Efficient Visible-Light-Driven Water Splitting Photocatalysts for Hydrogen Evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.B.; Song, J.T.; Oh, J.; Kim, S.H. Atomic Layer Deposited Molybdenum Disulfide on Si Photocathodes for Highly Efficient Photoelectrochemical Water Reduction Reaction. J. Mater. Chem. A 2017, 5, 3304–3310. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.Y.; Ma, F.X.; Hu, S.P.; Zhang, Y.W.; Zhen, L. Monodisperse SnS2 Nanosheets for High-Performance Photocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2014, 6, 22370–22377. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, F.; Shifa, T.A.; Liu, K.; Huang, Y.; Liu, Q.; Jiang, C.; He, J. Carbon Dots Decorated Vertical SnS2 Nanosheets for Efficient Photocatalytic Oxygen Evolution. Appl. Phys. Lett. 2016, 109, 053905. [Google Scholar] [CrossRef]
- Tu, J.R.; Shi, X.F.; Lu, H.W.; Yang, N.X.; Yuan, Y.J. Facile Fabrication of SnS2 Quantum Dots for Photoreduction of Aqueous Cr(VI). Mater. Lett. 2016, 185, 303–306. [Google Scholar] [CrossRef]
- Liu, J.H.; Huang, G.F.; Huang, W.Q.; Miao, H.; Zhou, B.X. Morphology-Controlled SnS2 Nanostructures Synthesized by Refluxing Method with High Photocatalytic Activity. Mater. Lett. 2015, 161, 480–483. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Chen, Z.; He, M.; Xie, M.; Liu, J.; Xu, H.; Huang, S.; Li, H. Different Morphologies SnS2 Supported on 2D G-C3N4 for Excellent and Stable Visible Light Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2018, 6, 5132–5141. [Google Scholar] [CrossRef]
- Lin, Y.T.; Shi, J.B.; Chen, Y.C.; Chen, C.J.; Wu, P.F. Synthesis and Characterization of Tin Disulfide (SnS2) Nanowires. Nanoscale Res. Lett. 2009, 4, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, E.; Hu, H.; Ouyang, S.; Xu, H.; Wang, D. Surfactant-Free Synthesis of Single Crystalline SnS2 and Effect of Surface Atomic Structure on the Photocatalytic Property. Int. J. Photoenergy 2014, 2014, 1–7. [Google Scholar]
- Zhao, W.; Wei, Z.; Ma, L.; Liang, J.; Zhang, X. Ag2S Quantum Dots Based on Flower-Like SnS2 as Matrix and Enhanced Photocatalytic Degradation. Materials 2019, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Cho, M.H. Growth of Three-Dimensional Flower-Like SnS2 on G-C3N4 Sheets as an Efficient Visible-Light Photocatalyst, Photoelectrode, and Electrochemical Supercapacitance Material. Sustain. Energ. Fuels 2017, 1, 510–519. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, M.; Hood, Z.D.; Pan, J.; Meng, Y.S.; Chi, M. Self-Assembled Framework Formed during Lithiation of SnS2 Nanoplates Revealed by in Situ Electron Microscopy. Acc. Chem. Res. 2017, 50, 1513–1520. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Mao, J.; Zhu, Y.; Fan, X.; Gao, T.; Mignerey, A.C.; Wang, C. Solid-State Fabrication of SnS2/C Nanospheres for High-Performance Sodium Ion Battery Anode. ACS Appl. Mater. Interfaces 2015, 7, 11476–11481. [Google Scholar] [CrossRef]
- Kovacic, M.; Katic, J.; Kusic, H.; Bozic, A.L.; Hukovic, M.M. Elucidating the Photocatalytic Behavior of TiO2-SnS2 Composites Based on Their Energy Band Structure. Materials 2018, 11, 1041. [Google Scholar] [CrossRef]
- Song, H.S.; Li, S.L.; Gao, L.; Xu, Y.; Ueno, K.; Tang, J.; Cheng, Y.B.; Tsukagoshi, K. High-Performance Top-Gated Monolayer SnS2 Field-Effect Transistors and Their Integrated Logic Circuits. Nanoscale 2013, 5, 9666–9670. [Google Scholar] [CrossRef]
- Burton, L.A.; Whittles, T.; Hesp, D.; Linhart, W.M.; Skelton, J.M.; Hou, B.; Webster, R.; O’Dowd, G.; Christian, R.; Cherns, D.; et al. Electronic and Optical Properties of Single Crystal SnS2: An Earth-Abundant Disulfide Photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Hasan, T.; Chen, X.; Zheng, W.; Feng, W.; Jia, D.; Zhou, Y.; Hu, P. Vertically Aligned Two-Dimensional SnS2 Nanosheets with a Strong Photon Capturing Capability for Efficient Photoelectrochemical Water Splitting. J. Mater. Chem. A 2017, 5, 1989–1995. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Y.; Wang, Z.; Zhang, J.; Chen, X.; Dai, M.; Jia, D.; Zhou, Y.; Li, Z.; Hu, P. Efficiently Synergistic Hydrogen Evolution Realized by Trace Amount of Pt-Decorated Defect-Rich SnS2 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 37750–37759. [Google Scholar] [CrossRef]
- Ham, G.; Shin, S.; Park, J.; Choi, H.; Kim, J.; Lee, Y.-A.; Seo, H.; Jeon, H. Tuning the Electronic Structure of Tin Sulfides Grown by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2013, 5, 8889–8896. [Google Scholar] [CrossRef]
- Ham, G.; Shin, S.; Park, J.; Lee, J.; Choi, H.; Lee, S.; Jeon, H. Engineering the Crystallinity of Tin Disulfide Deposited at Low Temperatures. RSC Adv. 2016, 6, 54069–54075. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A Brief Review of Atomic Layer Deposition: From Fundamentals to Applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Zhuiykov, S. Development of Ultra-Thin 2D Semiconductors by Atomic Layer Deposition, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 251–294. [Google Scholar]
- Buchholz, D.B.; Liu, J.; Marks, T.J.; Zhang, M.; Chang, R.P.H. Control and Characterization of the Structural, Electrical, and Optical Properties of Amorphous Zinc−Indium−Tin Oxide Thin Films. ACS Appl. Mater. Interfaces 2009, 1, 2147–2153. [Google Scholar] [CrossRef]
- Shang, M.; Qi, H.; Du, C.; Huang, H.; Wu, S.; Zhang, J.; Song, W. One-Step Electrodeposition of High-Quality Amorphous Molybdenum Sulfide/RGO Photoanode for Visible-Light Sensitive Photoelectrochemical Biosensing. Sens. Actuators B Chem. 2018, 266, 71–79. [Google Scholar] [CrossRef]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous Molybdenum Sulfide Films as Catalysts for Electrochemical Hydrogen Production in Water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Heo, J.; Noh, W.; Hock, A.S.; Gordon, R.G. Atomic Layer Deposition of Tin Monosulfide Thin Films (P1-31). Adv. Energy Mater. 2011, 1, 1116–1125. [Google Scholar] [CrossRef]
- Price, L.; Parkin, I.; Hardy, A.; Clark, R. Atmospheric Pressure Chemical Vapor Deposition of Tin Sulfides (SnS, Sn2S3, and SnS2) on Glass. Chem. Mater. 1999, 11, 1792–1799. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, M.J.; Heo, H.; Sung, J.H.; Kim, K.; Hwang, H.; Jo, M.H. Deterministic Two-Dimensional Polymorphism Growth of Hexagonal n-Type SnS2 and Orthorhombic p-Type SnS Crystals. Nano Lett. 2015, 15, 3703–3708. [Google Scholar] [CrossRef]
- Ohring, M. Materials Science of Thin Films, 2nd ed.; Academic Press, A division of Harcourt, Inc.: London, UK, 2018; pp. 251–294. [Google Scholar]
- Majumder, S.; Mendhe, A.C.; Kim, D.; Sankapal, B.R. CdO Nanonecklace: Effect of Air Annealing on Performance of Photo Electrochemical Cell. J. Alloys Compd. 2019, 788, 75–82. [Google Scholar] [CrossRef]
- Devika, M.; Reddy, K.T.R.; Reddy, N.K.; Ramesh, K.; Ganesan, R.; Gopal, E.S.R.; Gunasekhar, K.R. Microstructure Dependent Physical Properties of Evaporated Tin Sulfide Films. J. Appl. Phys. 2006, 100, 023518. [Google Scholar] [CrossRef]
- Matmin, J.; Jalani, M.A.; Osman, H.; Omar, Q.; Ab’lah, N.; Elong, K.; Kasim, M.F. Photochemical Synthesis of Nanosheet Tin Di/Sulfide with Sunlight Response on Water Pollutant Degradation. Nanomaterials 2019, 9, 264. [Google Scholar] [CrossRef]
- Huang, P.-C.; Shen, Y.-M.; Brahma, S.; Shaikh, M.O.; Huang, J.-L.; Wang, S.-C. SnSx (x = 1, 2) Nanocrystals as Effective Catalysts for Photoelectrochemical Water Splitting. Catalysts 2017, 7, 252. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.; Pei, X.; Luo, X.; Luo, S. Facile in Situ Hydrothermal Synthesis of G-C3N4/SnS2 Composites with Excellent Visible-Light Photocatalytic Activity. Mater. Chem. Phys. 2017, 189, 169–175. [Google Scholar] [CrossRef]
- Kment, S.; Cada, M.; Hubicka, Z.; Krysa, J.; Kmentova, H.; Olejnicek, J.; Cilova, Z.Z.; Zboril, R. Role of Ion Bombardment, Film Thickness and Temperature of Annealing on PEC Activity of Very-Thin Film Hematite Photoanodes Deposited by Advanced Magnetron Sputtering. Int. J. Hydrog. Energy 2016, 41, 11547–11557. [Google Scholar] [CrossRef]
- Saracco, G.; Barbero, G.; Hernández, S.; Alexe-Ionescu, A.L. Non-Monotonic Dependence of the Current Density on the Thickness of the Photoactive Layer. J. Electroanal. Chem. 2017, 788, 61–65. [Google Scholar] [CrossRef]
- Hernández, S.; Saracco, G.; Barbero, G.; Alexe-Ionescu, A.L. Role of the Electrode Morphology on the Optimal Thickness of BiVO4 Anodes for Photoelectrochemical Water Splitting Cells. J. Electroanal. Chem. 2017, 799, 481–486. [Google Scholar] [CrossRef]
- Hieu, H.N.; Dung, N.Q.; Kim, J.; Kim, D. Urchin-Like Nanowire Array: A Strategy for High-Performance ZnO-Based Electrode Utilized in Photoelectrochemistry. Nanoscale 2013, 5, 5530–5538. [Google Scholar] [CrossRef]
- Shi, J.; Hara, Y.; Sun, C.; Anderson, M.A.; Wang, X. Three-Dimensional High-Density Hierarchical Nanowire Architecture for High-Performance Photoelectrochemical Electrodes. Nano Lett. 2011, 11, 3413–3419. [Google Scholar] [CrossRef]
- He, S.; Meng, Y.; Cao, Y.; Huang, S.; Yang, J.; Tong, S.; Wu, M. Hierarchical Ta-Doped TiO2 Nanorod Arrays with Improved Charge Separation for Photoelectrochemical Water Oxidation under FTO Side Illumination. Nanomaterials 2018, 8, 983. [Google Scholar] [CrossRef]
- Parkinson, B. On the Efficiency and Stability of Photoelectrochemical Device. Acc. Chem. Res. 1984, 17, 431–437. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Leu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; et al. Freestanding Tin Disulfide Single-Layers Realizing Efficient Visible-Light Water Splitting. Angew. Chemie Int. Ed. 2012, 51, 8727–8731. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting —Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]





| Deposition Temperature (°C) | Crystal Structure | Optical Band Gap (eV) |
|---|---|---|
| 60 | SnS2 Amorphous | 2.80 |
| 100 | SnS2 Amorphous | 2.68 |
| 140 | SnS2 Amorphous | 2.52 |
| 180 | SnS orthorhombic | 1.78 |
| No. | Working Electrode Structure | Photocurrent Density (µA/cm2) | Reference Electrode (Applied Potential) | Solution | Irradiation | Reference (Year) |
|---|---|---|---|---|---|---|
| 1 | Glass/ITO/SnS2 (single-layer) | 2750 | Ag/AgCl (1.0 V) | 0.5 M Na2SO4 | 300 W Xe lamp irradiation (λ > 420 nm) | 47 (2012) |
| 2 | Glass/ITO/SnS2 (bulk) | <5 | ||||
| 3 | Glass/ITO/SnS2 NSs (with PVP) | 11.7 | SCE (0.8 V) | 0.5 M Na2SO4 | 300 W Xe lamp illumination (λ > 420 nm) | 8 (2014) |
| 4 | Glass/ITO/SnS2 NSs (without PVP) | 3.7 | ||||
| 5 | Glass/ITO/SnS2 NSs (water and 0.5 g PVP) | 2.3 | ||||
| 6 | Glass/FTO/Ni/SnS2/C (FNSC) | 38.6 | SCE (0 V) | 0.5 M Na2SO4 | Xenon lamp, 100 mW cm-2 (λ > 420 nm) | 9 (2016) |
| 7 | Glass/FTO/Ni/SnS2 (FNS) | 19.8 | ||||
| 8 | Glass/FTO/SnS2 (FS) | 16.6 | ||||
| 9 | CC⊥SnS2 | 1920 | RHE (1.4 V) | 0.5 M Na2SO4 | AM 1.5 G 100 mW cm−2 solar light | 22 (2017) |
| 10 | Glass/FTO⊥SnS2 | 1730 | ||||
| 11 | Glass/FTO//SnS2 | 910 | ||||
| 12 | Glass/Cr/Au/SnS2 | −2 | Ag/AgCl (−0.4 V) | 0.25 M H2SO4 | Simulated AM 1.5 sunlight (100 mW cm−2) | 38 (2017) |
| 13 | Glass/Cr/Au/SnS2 | 195 | Ag/AgCl (0.8 V) | |||
| 14 | g-C3N4/SnS2 nanoparticle | 13 | Ag/AgCl (1.0 V) | 0.5 M Na2S | 300 W xenon lamp (λ > 400 nm) | 12 (2018) |
| 15 | g-C3N4/SnS2 NS | 10 | ||||
| 16 | g-C3N4/3D flower-like SnS2 | 7.5 | ||||
| 17 | Glass/FTO/SnS2 (amorphous) | −35.1 | SCE (−0.6 V) | 0.25 M H2SO4 | Simulated AM 1.5 sunlight (100 mW cm−2) | This study |
| 18 | Glass/FTO/SnS2 (amorphous) | 51.5 | SCE (0.8 V) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Hien, T.T.; Kim, D.; Chang, H.S. Enhancement in Photoelectrochemical Performance of Optimized Amorphous SnS2 Thin Film Fabricated through Atomic Layer Deposition. Nanomaterials 2019, 9, 1083. https://doi.org/10.3390/nano9081083
Hu W, Hien TT, Kim D, Chang HS. Enhancement in Photoelectrochemical Performance of Optimized Amorphous SnS2 Thin Film Fabricated through Atomic Layer Deposition. Nanomaterials. 2019; 9(8):1083. https://doi.org/10.3390/nano9081083
Chicago/Turabian StyleHu, Weiguang, Truong Thi Hien, Dojin Kim, and Hyo Sik Chang. 2019. "Enhancement in Photoelectrochemical Performance of Optimized Amorphous SnS2 Thin Film Fabricated through Atomic Layer Deposition" Nanomaterials 9, no. 8: 1083. https://doi.org/10.3390/nano9081083
APA StyleHu, W., Hien, T. T., Kim, D., & Chang, H. S. (2019). Enhancement in Photoelectrochemical Performance of Optimized Amorphous SnS2 Thin Film Fabricated through Atomic Layer Deposition. Nanomaterials, 9(8), 1083. https://doi.org/10.3390/nano9081083