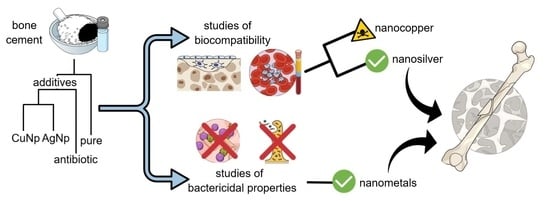
Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cement Preparation
2.2. Antibacterial Properties Testing on Orthopaedic Bacteria
2.2.1. Bacterial Growth Inhibition
2.2.2. Inhibition of Bacterial Adhesion to the Surface
2.3. Cytocompatibility Testing on Blood Components
2.3.1. Blood Collection and Preparation
2.3.2. In Vitro Hemolysis Assay and Evaluation of Erythrocyte Morphology
2.3.3. Platelet Aggregation
2.3.4. MTT Platelet Viability Test
2.4. Cytocompatibility Testing in Cultures of Dental Pulp Stem Cells (DPSC)
2.4.1. DPSC Collection and Preparation
2.4.2. MTS Cell Viability Test
2.4.3. Evaluation of DPSC Cells Morphology
2.4.4. Adhesion Assessment of DPSC Cells to the Surface
2.5. Statistical Method
3. Results
3.1. Antibacterial Properties Testing on Orthopedic Bacteria
3.1.1. Bacterial Growth Inhibition
3.1.2. Inhibition of Adhesion of Bacteria to the Surface
3.2. Cytocompatibility of Bioactive Bone Cements with Erythrocytes and Blood Platelets
3.2.1. Effect of Bone Cement Modifications on In Vitro Hemolysis and Erythrocyte Morphology
3.2.2. Effect of Bone Cement Modifications on In Vitro Platelet Aggregation and Their Viability
3.3. Cytocompatibility of Bioactive Bone Cement with Dental Pulp Stem Cells
3.3.1. MTS Cell Viability Test
3.3.2. Evaluation of DPSC Cells Morphology
3.3.3. Adhesion Assessment of DPSC Cells to the Material Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radha, G.; Balakumar, S.; Venkatesan, B.; Vellaichamy, E. A novel nano-hydroxyapatite—PMMA hybrid scaffolds adopted by conjugated thermal induced phase separation (TIPS) and wet-chemical approach: Analysis of its mechanical and biological properties. Mater. Sci. Eng. C 2017, 73, 164–172. [Google Scholar]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curatolo, C.J.; Anderson, M.R. Bone cement implantation syndrome. In Decision-Making in Orthopedic and Regional Anesthesiology: A Case-Based Approach; Anderson, M.R., Wilson, S.H., Rosenblatt, M.A., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 118–122. [Google Scholar]
- Slane, J.; Vivanco, J.; Ebenstein, D.; Squire, M.; Ploeg, H.L. Multiscale characterization of acrylic bone cement modified with functionalized mesoporous silica nanoparticles. J. Mech. Behav. Biomed. Mater. 2014, 37, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gu, J.; Shah, L.A.; Siddiq, M.; Hu, J.; Cai, X.; Yang, D. Bone cement based on vancomycin loaded mesoporous silica nanoparticle and calcium sulfate composites. Mater. Sci. Eng. C 2015, 49, 210–216. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Hoess, A.; López, A.; Engqvist, H.; Ott, M.K.; Persson, C. Comparison of a quasi-dynamic and a static extraction method for the cytotoxic evaluation of acrylic bone cements. Mater. Sci. Eng. C 2016, 62, 274–282. [Google Scholar] [CrossRef]
- Robo, C.; Hulsart-Billström, G.; Nilsson, M.; Persson, C. In vivo response to a low-modulus PMMA bone cement in an ovine model. Acta Biomater. 2018, 72, 362–370. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef]
- Cenni, E.; Granchi, D.; Vancini, M.; Pizzoferrato, A. Platelet release of transforming growth factor-β and β-thromboglobulin after in vitro contact with acrylic bone cements. Biomaterials 2002, 23, 1479–1484. [Google Scholar] [CrossRef]
- Matus, F.; Vilos, C.; Cisterna, B.A.; Fuentes, E.; Palomo, I. Nanotechnology and primary hemostasis: Differential effects of nanoparticles on platelet responses. Vasc. Pharmacol. 2018, 101, 1–8. [Google Scholar] [CrossRef]
- Fröhlich, E. Hemocompatibility of inhaled environmental nanoparticles: Potential use of in vitro testing. J. Hazard. Mater. 2017, 336, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Zamborsky, R.; Svec, A.; Bohac, M.; Kilian, M.; Kokavec, M. Infection in bone allograft transplants. Exp. Clin. Transplant. 2016, 14, 484–490. [Google Scholar] [PubMed]
- Miola, M.; Bistolfi, A.; Valsania, M.C.; Bianco, C.; Fucale, G.; Verné, E. Antibiotic-loaded acrylic bone cements: An in vitro study on the release mechanism and its efficacy. Mater. Sci. Eng. C 2013, 33, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Rzhepishevska, O.; Grenho, L.; Malheiros, D.; Gonçalves, L.; Almeida, A.J.; Jordão, L.; Ribeiro, I.; Ramstedt, M.; Gomes, P.; et al. Levofloxacin-loaded bone cement delivery system: Highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int. J. Pharm. 2017, 532, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Sanz-Ruiz, P.; Abenojar, J.; Vaquero-Martín, J.; Forriol, F.; Del Real, J.C. Evaluation of Elution and Mechanical Properties of High-Dose Antibiotic-Loaded Bone Cement: Comparative “In Vitro” Study of the Influence of Vancomycin and Cefazolin. J. Arhroplasty 2015, 30, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Frutos, G.; Pastorr, J.Y.; Martinez, N.; Virto, M.R.; Torrado, S. Influence of lactose addition to gentamicin-loaded acrylic bone cement on the kinetics of release of the antibiotic and the cement properties. Acta Biomater. 2010, 6, 804–811. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Dias, S.D.; Otero-González, A.J.; Franco, O.L. Antimicrobial magnetic nanoparticles based-therapies for controlling infectious diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Muñoz, L.; Tamayo, L.; Gulppi, M.; Rabagliati, F.; Flores, M.; Urzúa, M.; Azócar, M.; Zagal, J.H.; Encinas, M.V.; Zhou, X.; et al. Surface functionalization of an aluminum alloy to generate an antibiofilm coating based on poly(methyl methacrylate) and silver nanoparticles. Molecules 2018, 23, 2747. [Google Scholar] [CrossRef]
- Paiva, L.; Fidalgo, T.; Da Costa, L.; Maia, L.; Balan, L.; Anselme, K.; Ploux, L.; Thiré, R. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Burdusel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L., Jr. A Grain of Salt: Metallic and Metallic Oxide Nanoparticles as the New Antimicrobials. JSM Nanotechnol. Nanomed. 2014, 2, 1026. [Google Scholar]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz-Dosque, M.; Pérez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol-gel method. Mater. Sci. Eng. C 2013, 33, 3795–3801. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Savelyev, Y.; Gonchar, A.; Movchan, B.; Gornostay, A.; Vozianov, S.; Rudenko, A.; Rozhnova, R.; Travinskaya, T. Antibacterial polyurethane materials with silver and copper nanoparticles. Mater. Today Proc. 2017, 4, 87–94. [Google Scholar] [CrossRef]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol–gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 25001. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, G.S.; Kontonasaki, E.; Theocharidou, A.; Bakopoulou, A.; Bousnaki, M.; Hadjichristou, C.; Papachristou, E.; Papadopoulou, L.; Kantiranis, N.A.; Chrissafis, K.; et al. Sol-Gel Derived Mg-Based Ceramic Scaffolds Doped with Zinc or Copper Ions: Preliminary Results on Their Synthesis, Characterization, and Biocompatibility. Int. J. Biomater. 2016, 2016, 3858301. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.F.; Alegria-Acevedo, L.F.; Mendez-Bauer, L.; Bermudez, J.; Davila-Sanchez, A.; Buvinic, S.; Hernandez-Moya, N.; Reis, A.; Loguercio, A.D.; Faragao, P.V.; et al. Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J. Dent. 2019, 82, 45–55. [Google Scholar] [CrossRef]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Hoque, N.A.; Thakur, P.; Das, S. A facile vacuum assisted synthesis of nanoparticle impregnated hydroxyapatite composites having excellent antimicrobial properties and biocompatibility. Ceram. Int. 2018, 44, 1066–1077. [Google Scholar] [CrossRef]
- Shen, S.-C.; Letchmanan, K.; Chow, P.S.; Tan, R.B.H. Antibiotic elution and mechanical property of TiO2 nanotubes functionalized PMMA-based bone cements. J. Mech. Behav. Biomed. Mater. 2019, 91, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Li, Y.; Morris, T. Micro and nano MgO particles for the improvement of fracture toughness of bone-cement interfaces. J. Biomech. 2013, 46, 1035–1039. [Google Scholar] [CrossRef]
- Khaled, S.; Charpentier, P.A.; Rizkalla, A.S. Synthesis and characterization of poly(methyl methacrylate)-based experimental bone cements reinforced with TiO2-SrO nanotubes. Acta Biomater. 2010, 6, 3178–3186. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef]
- Cierech, M.; Osica, I.; Kolenda, A.; Wojnarowicz, J.; Szmigiel, D.; Łojkowiski, W.; Kurzydłowski, K.; Ariga, K.; Mierzwińska-Nastalska, E. Mechanical and Physicochemical Properties of Newly Formed ZnO-PMMA Nanocomposites for Denture Bases. Nanomaterials 2018, 8, 305. [Google Scholar] [CrossRef]
- Russo, T.P.; Gloria, A.B.; De Santis, R.; D’Amora, U.; Barbaric, K.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The future in dental care for eldery edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Slane, J.; Vivanco, J.; Rose, W.; Ploeg, H.L.; Squire, M. Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Mater. Sci. Eng. C 2015, 48, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, O.; Goncharova, I.; Rimpelova, S.; Kolarova, K.; Svanda, J.; Svorcik, V. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mater. Sci. Eng. C 2015, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- International Standard ISO 5833. Implants for Surgery—Acrylic Resin Cements; International Standard ISO: Geneva, Switzerland, 2002. [Google Scholar]
- Wekwejt, M.; Moritz, M.; Świeczko-Żurek, B.; Pałubicka, A. Biomechanical testing of bioactive bone cements—A comparison of the impact of modifiers: Antibiotics and nanometals. Polym. Test. 2018, 70, 234–243. [Google Scholar] [CrossRef]
- Wekwejt, M.; Pałubicka, A. Antibacterial evaluation of bioactive modifiers of bone cements: Antibiotics, nanometals and chitosan. Eur. J. Med. Technol. 2018, 3, 6–10. [Google Scholar]
- Clinical & Laboratory Standards Institute. M07: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- International Standard for Blood Banks & Blood Transfusion Services; NACO: New Delhi, India, 2007.
- Henkelman, S.; Rakhorst, G.; Blanton, J.; Van Oeveren, W. Standardization of incubation conditions for hemolysis testing of biomaterials. Mater. Sci. Eng. C 2019, 29, 1650–1654. [Google Scholar] [CrossRef]
- Wang, J.; Green, P.S.; Simpkins, J.W. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J. Neurochem. 2001, 77, 804–811. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Montufar, E.B. Cements as bone repair materials. In Bone Repair Biomaterials; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 233–271. [Google Scholar]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Nussler, A.; Sajadian, S.O. Adult Stem Cells. Stem Cell Rev. 2015, 1553, 3–23. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Preparation of Cells and Tissues for Fluorescence Microscopy. In Basic Methods in Microscopy: Protocols and Concepts from Cells: A Laboratory Manual; Spectro, D.L., Goldman, R.D., Eds.; Cold Spring Harbor Laboratory Press: Laurel Hollow, NY, USA, 2005. [Google Scholar]
- Parameswaran, S.; Verma, R.S. Scanning electron microscopy preparation protocol for differentiated stem cells. Anal. Biochem. 2011, 416, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Class II Special Controls Guidance Document: Polymethylmethacrylate (PMMA) Bone Cement—Guidance for Industry and FDA. Available online: https://fda.gov/regulatory-information/search-fda-guidance-documents/class-ii-special-controls-guidance-document-polymethylmethacrylate-pmma-bone-cement-guidance (accessed on 22 July 2019).
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Martínez-Moreno, J.; Mura, C.; Merino, V.; Nácher, A.; Climente, M.; Merino-Sanjuán, M. Study of the Influence of Bone Cement Type and Mixing Method on the Bioactivity and the Elution Kinetics of Ciprofloxacin. J. Arthroplasty 2015, 30, 1243–1249. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Prokopovich, P.; Köbrick, M.; Brousseau, E.; Perni, S. Potent antimicrobial activity of bone cement encapsulating silver nanoparticles capped with oleic acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Prokopovich, P.; Leech, R.; Carmalt, C.J.; Parkin, I.P.; Persi, S. A novel bone cement impregnated with silver-tiopronin nanoparticles: Its antimicrobial, cytotoxic, and mechanical properties. Int. J. Nanomed. 2013, 8, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Pauksch, L.; Hartmann, S.; Szalay, G.; Alt, V.; Lips, K.S. In vitro assessment of nanosilver-functionalized PMMA bone cement on primary human mesenchymal stem cells and osteoblasts. PLoS ONE 2014, 9, e114740. [Google Scholar] [CrossRef]
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 25518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrochenko, P.E.; Zheng, J.; Casey, B.J.; Bayati, M.R.; Narayan, R.J.; Goering, P.L. Nanosilver-PMMA composite coating optimized to provide robust antibacterial efficacy while minimizing human bone marrow stromal cell toxicity. Toxicol. Vitr. 2017, 44, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Zhu, C.; Fang, X.; Ma, B.; Wan, L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, L.; Tang, H.; Xu, M.; Wang, Y.; Yang, X.; Chen, H.; Li, Y.; Ye, G.; Shi, F.; et al. The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats. Int. J. Mol. Sci. 2019, 20, 1469. [Google Scholar] [CrossRef] [PubMed]
- Jaidev, L.R.; Kumar, S.; Chatterjee, K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf. B 2017, 159, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Robatto, B.M.; López-Alvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; Gonzalez, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Hu, L.X.; Hu, S.F.; Rao, M.; Yang, J.; Lei, H.; Duan, Z.; Xia, W.; Zhu, C. Studies of acute and subchronic systemic toxicity associated with a copper/low-density polyethylene nanocomposite intrauterine device. Int. J. Nanomed. 2018, 13, 4913–4926. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. Part A 2014, 102, 3556–3561. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Sawant, S.N.; Selvaraj, V.; Prabhawathi, V.; Doble, M. Antibiofilm Properties of Silver and Gold Incorporated PU, PCLm, PC and PMMA Nanocomposites under Two Shear Conditions. PLoS ONE 2013, 8, e63311. [Google Scholar] [CrossRef] [PubMed]
- Moojen, D.J.F.; Vogely, H.C.; Fleer, A.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J.A. No efficacy of silver bone cement in the prevention of methicillin-sensitive Staphylococcal infections in a rabbit contaminated implant bed model. J. Orthop. Res. 2009, 27, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Rosales, M.; Ávila-Orta, C.A.; Neira-Velázquez, M.G.; Ortega-Ortiz, H.; Hernández-Hernández, E.; Solís-Rosales, S.G.; España-Sánchez, B.L.; Gõnzalez-Morones, P.; Jímenez-Barrera, R.M.; Sánchez-Valdes, S.; et al. Effect of plasma modification of copper nanoparticles on their antibacterial properties. Plasma Process Polym 2014, 11, 685–693. [Google Scholar] [CrossRef]
- Anyaogu, K.C.; Fedorov, A.C.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 2008, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Balela, M.D.L.; Amores, K.L.S. Electroless deposition of copper nanoparticle for antimicrobial coating. Mater. Chem. Phys. 2019, 225, 393–398. [Google Scholar] [CrossRef]
- Miola, M.; Cochis, A.; Kumar, A.; Arciola, C.R.; Rimondini, L.; Verné, E. Copper-doped bioactive glass as filler for PMMA-based bone cements: Morphological, mechanical, reactivity, and preliminary antibacterial characterization. Materials 2018, 11, 961. [Google Scholar] [CrossRef]
- Khaaton, U.T.; Rao, N.G.V.S.; Mantravadi, K.M.; Ramanaviciene, A.; Ramanavivius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Aleksandr, L.; Alexander, P.; Olga, B.; Sergey, K.; Irena, G. Synthesis of antimicrobial AlOOH-Ag composite nanostructures by water oxidation of bimetallic Al-Ag nanoparticles. RSC Adv. 2018, 8, 36239–36244. [Google Scholar] [CrossRef]
- Kaygusuz, M.; Lkhagvajav, N.; Yasa, I.; Celik, E. Antimicrobial Nano-Ag-TiO2 coating for lining leather. Rom. Biotechnol. Lett. 2016, 21, 18866–18874. [Google Scholar]
- Sopjani, M.; Föller, M.; Haendeler, J.; Götz, F.; Lang, F. Silver ion-induced suicidal erythrocyte death. J Appl Toxicol 2009, 29, 531–536. [Google Scholar] [CrossRef]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2017, 107, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Chang, L.W.; Lin, P. Metal-Based Nanoparticles and the Immune System: Activation, Inflammation, and Potential Applications. BioMed Res. Int. 2015, 2015, 143720. [Google Scholar] [CrossRef] [PubMed]







| Chemical Composition | Unmodified Bone Cement/BC/ | Antibiotic-Loaded Bone Cement/BC A/ | Bone Cement Modified with Nanometals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| nanoAg/BC-NpAg/ | nanoCu/BC-NpCu/ | nanoAg & nanoCu/BC-NpAg+Cu/ | |||||||
| Powder Component (% w/w) | |||||||||
| Polymethyl methacrylate | 84.30 | 83.05 | 81.77 | 83.05 | 81.77 | 83.05 | 81.77 | 83.05 | |
| Barium sulfate | 13.00 | 12.80 | 12.61 | 12.80 | 12.61 | 12.80 | 12.61 | 12.80 | |
| Benzoyl peroxide | 2.70 | 2.65 | 2.62 | 2.65 | 2.62 | 2.65 | 2.62 | 2.65 | |
| Gentamicin sulphate | ------- | 1.50 | 3.00 | ------- | ------- | ------- | ------- | ------- | |
| NanoAg | ------- | ------- | ------ | 1.50 | 3.00 | ------- | ------- | 1.15 | 1.50 |
| NanoCu | ------- | ------- | ------ | ------- | ------ | 1.50 | 3.00 | 0.35 | |
| Liquid Component (% w/w) | |||||||||
| Methyl Methacrylate | 99.10 | ||||||||
| N,N-dimethyl-p-toluidine | 0.90 | ||||||||
| Hydroquinone | 75.00 | ||||||||
| McFarland Index | ||||||
|---|---|---|---|---|---|---|
| Time (h) | K | BC | BC-NpAg | BC-NpCu | BC-NpAg+Cu | BC-A |
| 0 | 0.5 ± 0.01 | |||||
| 0.5 | 0.68 ± 0.01 | 0.71 ± 0.02 | 0.68 ± 0.01 | 0.70 ± 0.02 | 0.60 ± 0.01 | 0.65 ± 0.01 |
| 2 | 1.93 ± 0.03 | 1.64 ± 0.01 | 1.10 ± 0.01 | 1.95 ± 0.01 | 0.77 ± 0.02 | 1.24 ± 0.01 |
| 4 | >4 | 3.48 ± 0.02 | 1.22 ± 0.02 | × | 0.79 ± 0.02 | 1.35 ± 0.02 |
| 6 | >4 | >4 | 1.29 ± 0.01 | × | 0.80 ± 0.03 | 1.48 ± 0.02 |
| 24 | >4 | >4 | 1.33 ± 0.02 * | × | 0.91 ± 0.03 * | 1.52 ± 0.02 * |
| K | BC | BC-NpAg | BC-NpCu | BC-A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2′ | 2 h | 2′ | 2 h | 2′ | 2 h | 2′ | 2 h | 2′ | 2 h | |
| Spontaneous aggregation (%) | 1 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 1 |
| Trombin-evoked early phase of aggregation (1 min) (%) | 43 ± 6 | 31 ± 8 | 39 ± 3 | 23 ± 11 | 33 ± 8 | 23 ± 6 | 28 ± 13 | 4 ± 2 * | 32 ± 14 | 10 ± 3 * |
| Thrombin-evoked late phase of aggregation (10 min) (%) | 76 ± 9 | 67 ± 7 | 74 ± 8 | 64 ± 10 | 70 ± 8 | 49 ± 9 | 69 ± 6 | 22 ± 17 * | 67 ± 13 | 39 ± 7 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wekwejt, M.; Michno, A.; Truchan, K.; Pałubicka, A.; Świeczko-Żurek, B.; Osyczka, A.M.; Zieliński, A. Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration. Nanomaterials 2019, 9, 1114. https://doi.org/10.3390/nano9081114
Wekwejt M, Michno A, Truchan K, Pałubicka A, Świeczko-Żurek B, Osyczka AM, Zieliński A. Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration. Nanomaterials. 2019; 9(8):1114. https://doi.org/10.3390/nano9081114
Chicago/Turabian StyleWekwejt, Marcin, Anna Michno, Karolina Truchan, Anna Pałubicka, Beata Świeczko-Żurek, Anna Maria Osyczka, and Andrzej Zieliński. 2019. "Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration" Nanomaterials 9, no. 8: 1114. https://doi.org/10.3390/nano9081114
APA StyleWekwejt, M., Michno, A., Truchan, K., Pałubicka, A., Świeczko-Żurek, B., Osyczka, A. M., & Zieliński, A. (2019). Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration. Nanomaterials, 9(8), 1114. https://doi.org/10.3390/nano9081114