Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
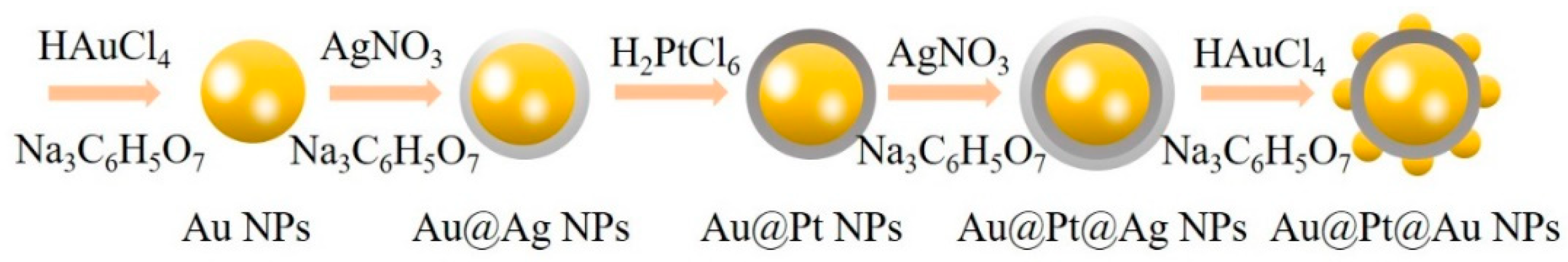
2.2. Synthesis of Au@Pt@Au Core-Shell NPs
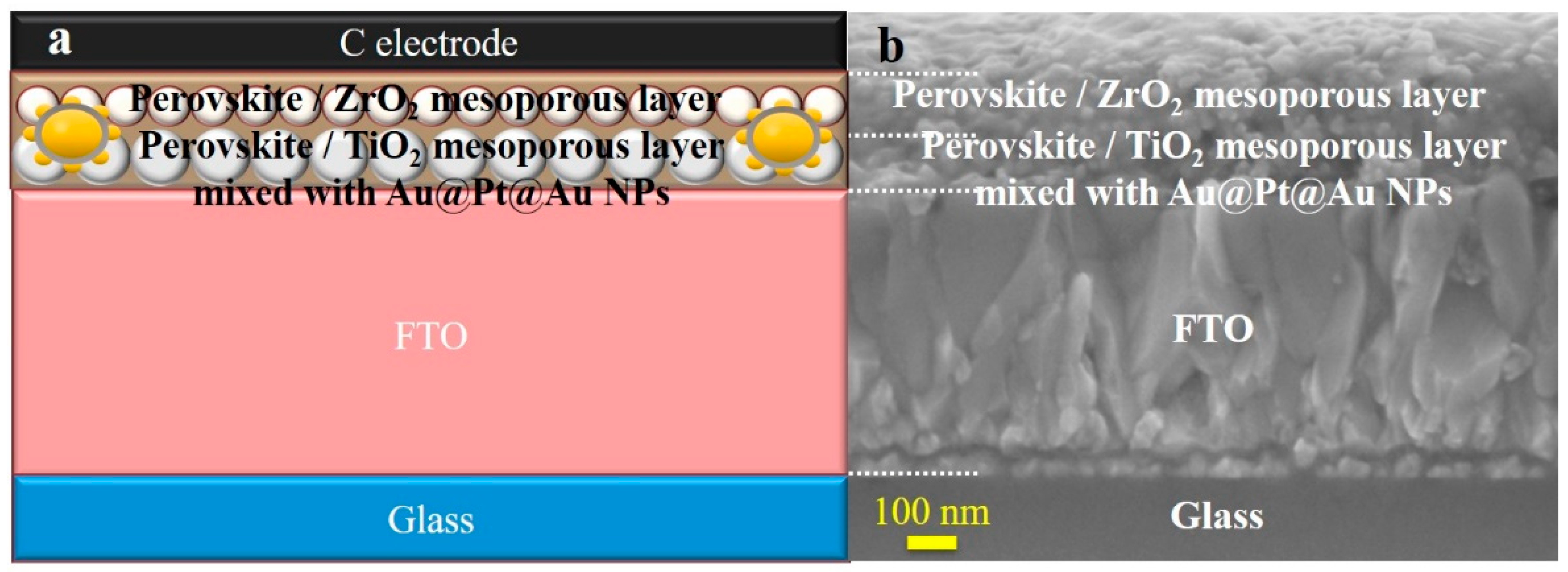
2.3. Cell Fabrication
2.4. Characterization
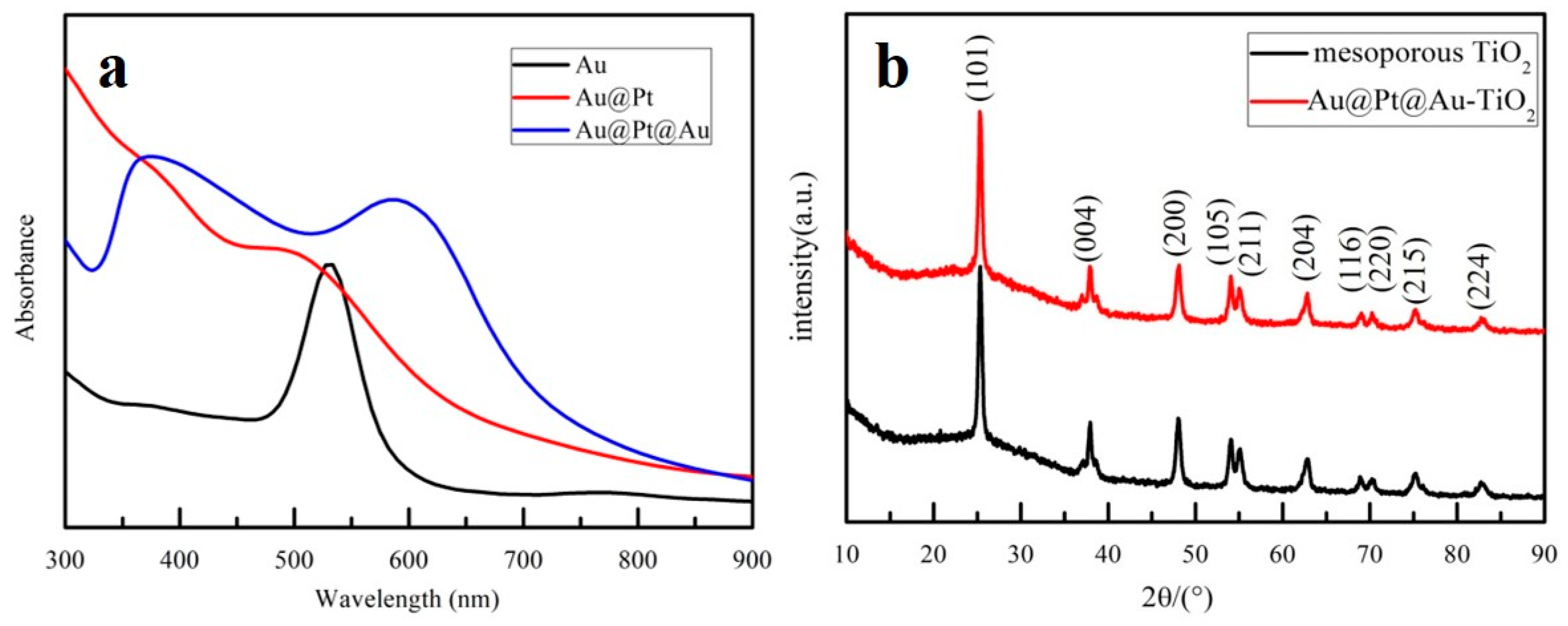
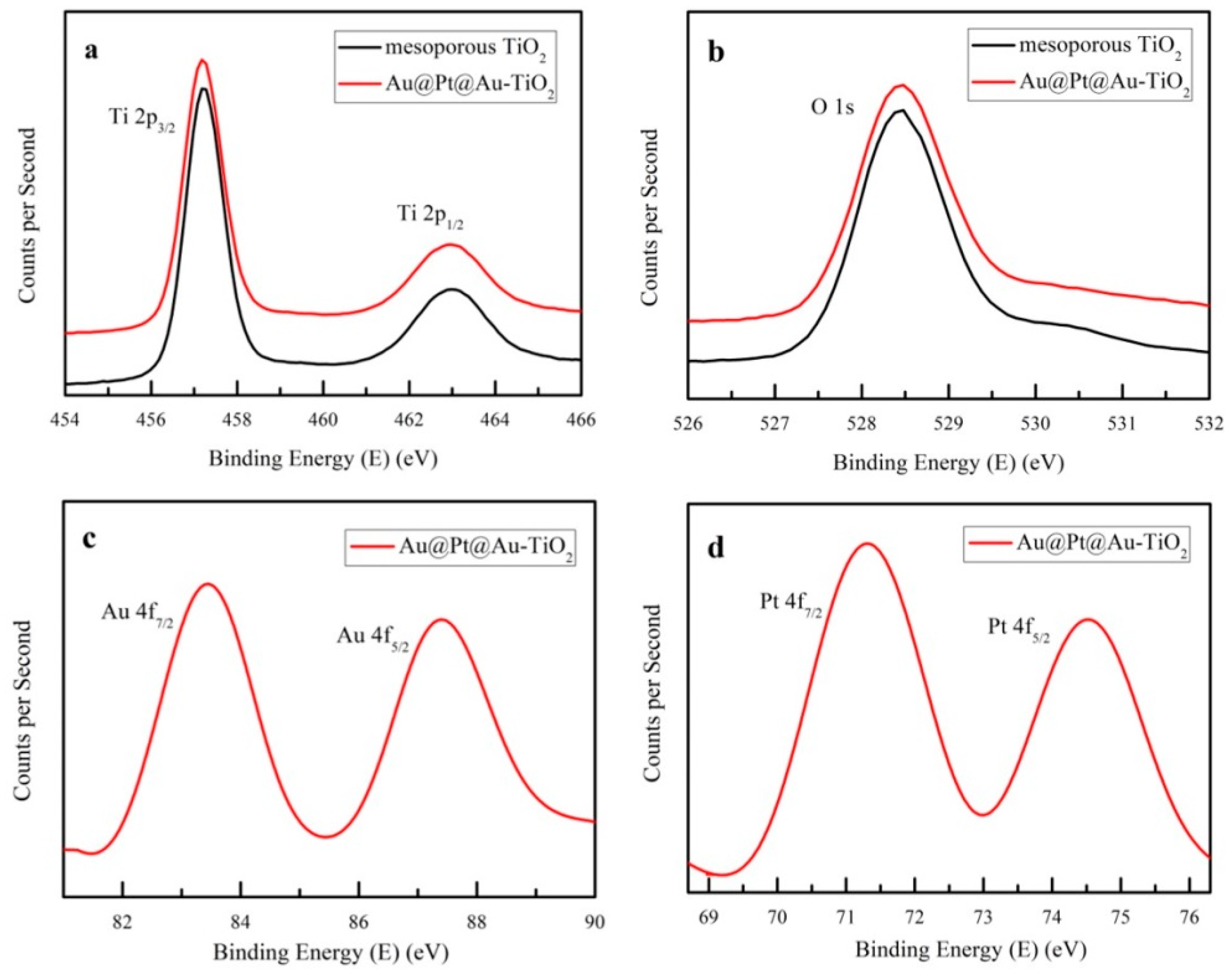
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumawat, N.K.; Tripathi, M.N.; Waghmare, U.; Kabra, D. Structural, optical, and electronic properties of wide bandgap perovskites: Experimental and theoretical investigations. J. Phys. Chem. A 2016, 120, 3917–3923. [Google Scholar] [CrossRef]
- Stranks, S.D.; Petrozza, A.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Lee, Y.H.; Luo, J.; Homphry-Baker, R.; Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Unraveling the reasons for efficiency loss in perovskite solar cells. Adv. Funct. Mater. 2015, 25, 3925–3933. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Yella, A.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Chen, K.; Wu, P.; Yang, W.Q.; Su, R.; Luo, D.; Yang, X.; Tu, Y.; Zhu, R.; Gong, Q. Low-dimensional perovskite interlayer for highly efficient lead-free formamidinium tin iodide perovskite solar cells. Nano Energy 2018, 49, 411–418. [Google Scholar] [CrossRef]
- Tu, Y.G.; Yang, X.Y.; Su, R.; Luo, D.; Cao, Y.; Zhao, L.; Liu, T.; Yang, W.; Zhang, Y.; Xu, Z.; et al. Diboron-assisted interfacial defect control strategy for highly efficient planar perovskite solar cells. Adv. Mater. 2018, 30, 1805085. [Google Scholar] [CrossRef]
- Luo, D.Y.; Yang, W.Q.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.H.; Zhou, Y.Y.; Li, Z.; Zhang, L.; Ju, M.-G.; Luo, D.; Yang, Y.; Yang, M.; Kim, D.H.; Yang, W.; et al. Stable formamidinium-based perovskite solar cells via in situ grain encapsulation. Adv. Energy Mater. 2018, 8, 1800232. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, M.; Wang, B.; Liu, N.; Ran, M.Q.; Yang, H.; Yang, Y.P. Improved photovoltaic properties of nominal composition CH3NH3Pb0.99Zn0.01I3 carbon-based perovskite solar cells. Opt. Express 2018, 26, 984–995. [Google Scholar] [CrossRef]
- Tonui, P.; Oseni, S.O.; Sharma, G.; Yan, Q.; Mola, G.T. Perovskites photovoltaic solar cells: An overview of current status. Renew. Sustain. Energy Rev. 2018, 91, 1025–1044. [Google Scholar] [CrossRef]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic-inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Yang, S.; Fu, W.; Zhang, Z.; Chen, H.; Li, C.-Z. Recent advances in perovskite solar cells: Efficiency, stability and lead-free perovskite. J. Mater. Chem. A 2017, 5, 11462–11482. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Abate, A. Perovskite solar cells go lead free. Joule 2017, 1, 659–664. [Google Scholar] [CrossRef]
- Ju, M.G.; Chen, M.; Zhou, Y.; Dai, J.; Ma, L.; Padture, N.P.; Zeng, X.C. Toward eco-friendly and stable perovskite materials for photovoltaics. Joule 2018, 2, 1231–1241. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward lead-free perovskite solar cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- Zhao, Z.; Gu, F.; Li, Y.; Sun, W.; Ye, S.; Rao, H.; Liu, Z.; Bian, Z.; Huang, C. Mixed-organic-cation tin iodide for lead-free perovskite solar cells with an efficiency of 8.12%. Adv. Sci. 2017, 4, 1700204. [Google Scholar] [CrossRef]
- Tress, W. Perovskite solar cells on the way to their radiative efficiency limitinsights into a success story of high open-circuit voltage and low recombination. Adv. Energy Mater. 2017, 7, 1602358. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 2019, 1803792. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Stratakis, E.; Kymakis, E. Nanoparticle-based plasmonic organic photovoltaic devices. Mater. Today 2013, 16, 133–146. [Google Scholar] [CrossRef]
- Petridis, C.; Savva, K.; Kymakis, E.; Stratakis, E. Laser generated nanoparticles based photovoltaics. J. Colloid Interface Sci. 2017, 489, 28–37. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Jost, M.; Albrecht, S.; Kegelmann, L.; Wolff, C.M.; Lang, F.; Lipovšek, B.; Krč, J.; Korte, L.; Neher, D.; Rech, B.; et al. Efficient light management by textured nanoimprinted layers for perovskite solar cells. ACS Photonics 2017, 4, 1232–1239. [Google Scholar] [CrossRef]
- Yang, H.; Jun, Z.; Yong, D.; Chen, S.; Zhang, C.; Dai, S. TiO2 sub-microsphere film as scaffold layer for efficient perovskite solar cells. ACS Appl. Mater. 2016, 1–13. [Google Scholar] [CrossRef]
- Hui, Z.; Mariia, K.; Johann, O.; Toudert, J.; Martorell, J. Natural random nano-texturing of the Au interface for light backscattering enhanced performance in perovskite solar cells. ACS Photonics 2018, 1–28. [Google Scholar] [CrossRef]
- Wei, J.; Xu, R.-P.; Li, Y.-Q.; Li, C.; Chen, J.-D.; Zhao, X.-D.; Xie, Z.-Z.; Lee, C.-S.; Zhang, W.-J.; Tang, J.-X. Enhanced light harvesting in perovskite solar cells by a bioinspired nanostructured back electrode. Communication 2017, 1700492. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, C.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Gaspera, D.E.; Peng, Y.; Hou, Q.; Spiccia, L.; Bach, U.; Jasieniak, J.J.; Cheng, Y.-B. Ultra-thin high efficiency semitransparent perovskite solar cells. Nano Energy 2015, 13, 249–257. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Yang, Y.; Zhao, L.; Qiao, Y.; Liu, W.; Liu, P.; Chen, M. Ag/nano-TiO2 composite compact film for enhanced performance of perovskite solar cells based on carbon counter electrodes. Appl. Phys. A 2017, 123, 628–636. [Google Scholar] [CrossRef]
- Li, S.H.; Zhu, X.Y.; Wang, B.; Qiao, Y.; Liu, W.; Yang, H.; Liu, N.; Chen, M.; Lu, H.; Yang, Y. Influence of Ag nanoparticles with different sizes and concentrations embedded in a TiO2 compact layer on the conversion efficiency of perovskite solar cells. Nanoscale Res. Lett. 2018, 13, 210–221. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.Y.; Li, S.H.; Chen, M.; Lu, H.; Yang, Y. Ag@SiO2 core-shell nanoparticles embedded in a TiO2 mesoporous layer substantially improve the performance of perovskite solar cells. Nanomaterials 2018, 8, 701. [Google Scholar] [CrossRef]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Gu, J.; Lan, G.; Jiang, Y.; Xu, Y.; Zhu, W.; Jin, C.; Zhang, Y. Shaped Pt-Ni nanocrystals with an ultrathin Pt-enriched shell derived from one-pot hydrothermal synthesis as active electrocatalysts for oxygen reduction. Nano Res. 2015, 8, 1480–1496. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Petridis, K.; Kymakis, E. Recent advances in plasmonic metal and rare-earth-element upconversion nanoparticle doped perovskite solar cells. J. Mater. Chem. A 2017, 5, 21604. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, Z.; Bai, S.; Xia, Z.; Xu, W.; Song, T.; Wu, H.; Xu, L.; Si, J.; Jin, Y.; et al. Hot-electron injection in a sandwiched TiOx-Au-TiOx structure for high-performance planar perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500038. [Google Scholar] [CrossRef]
- Huang, H.; Yang, Y. Preparation of silver nanoparticles in inorganic clay suspensions. Compos. Sci. Technol. 2008, 68, 2948–2953. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Size-controlled synthesis of nanoparticles. 1. “silver-only” aqueous suspensions via hydrogen reduction. J. Phys. Chem. B 2004, 108, 13948–13956. [Google Scholar] [CrossRef]
- Pietrobon, B.; Kitaev, V. Photochemical synthesis of monodisperse size-controlled silver decahedral nanoparticles and their remarkable optical properties. Chem. Mater. 2008, 20, 5186–5190. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via Lewis base adduct of lead(II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.-S.; Wang, H.-H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef]
- Barrows, A.T.; Pearson, A.J.; Kwak, C.K.; Dunbar, A.D.F.; Buckley, A.R.; Lidzey, D.G. Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition. Energy Environ. Sci. 2014, 7, 2944–2950. [Google Scholar] [CrossRef]
- Xie, W.; Herrmann, C.; Kompe, K.; Haase, M.; Schlücker, S. Synthesis of bifunctional Au/Pt/Au core/shell nanoraspberries for in situ SERS monitoring of platinum-catalyzed reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef]
- Qi, L.; Tang, C.; Zhang, L.; Yao, X.; Cao, Y.; Liu, L.; Gao, F.; Dong, L.; Chen, L. Influence of cerium modification methods on catalytic performance of Au/mordenite catalysts in CO oxidation. Appl. Catal. B Environ. 2012, 127, 234–245. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Li, C.; Hu, W.; Jing, P.; Wang, Q.; Zhang, J. Three-dimensionally ordered macroporous Au/CeO2-Co3O4 catalysts with nanoporous walls for enhanced catalytic oxidation of formaldehyde. Appl. Catal. B Environ. 2012, 127, 47–58. [Google Scholar] [CrossRef]
- Nie, R.; Wang, J.; Wang, L.; Qin, Y.; Chen, P.; Hou, Z. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon 2012, 50, 586–596. [Google Scholar] [CrossRef]
- Shi, X.; Ueno, K.; Takabayashi, N.; Misawa, H. Plasmon-enhanced photocurrent generation and water oxidation with a gold nanoisland-loaded titanium dioxide photoelectrode. J. Phys. Chem. C 2013, 117, 2494–2499. [Google Scholar] [CrossRef]
- Lee, J.E.; Bera, S.; Choi, Y.S.; Lee, W.I. Size-dependent plasmonic effects of M and M@SiO2, (M = Au or Ag) deposited on TiO2 in photocatalytic oxidation reactions. Appl. Catal. B Environ. 2017, 214, 15–22. [Google Scholar] [CrossRef]
- Xin, B.; Jing, L.; Ren, Z.; Wang, B.; Fu, H. Effects of simultaneously doped and deposited Ag on the photocatalytic activity and surface states of TiO2. J. Phys. Chem. B 2005, 109, 2805–2809. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Lee, K.E.; Scott, R.W.J.; Kelly, T.L. Plasmonic enhancement of dye sensitized solar cells in the red-to-near-infrared region using triangular core-shell Ag@SiO2 nanoparticles. Appl. Mater. Interfaces 2013, 5, 11044. [Google Scholar] [CrossRef]
- Severin, H.; Nakita, N.; Henry, S. Hysteresis index: A figure without merit for quantifying hysteresis in perovskite solar cells. ACS Energy Lett. 2018, 3, 2472–2476. [Google Scholar] [CrossRef]

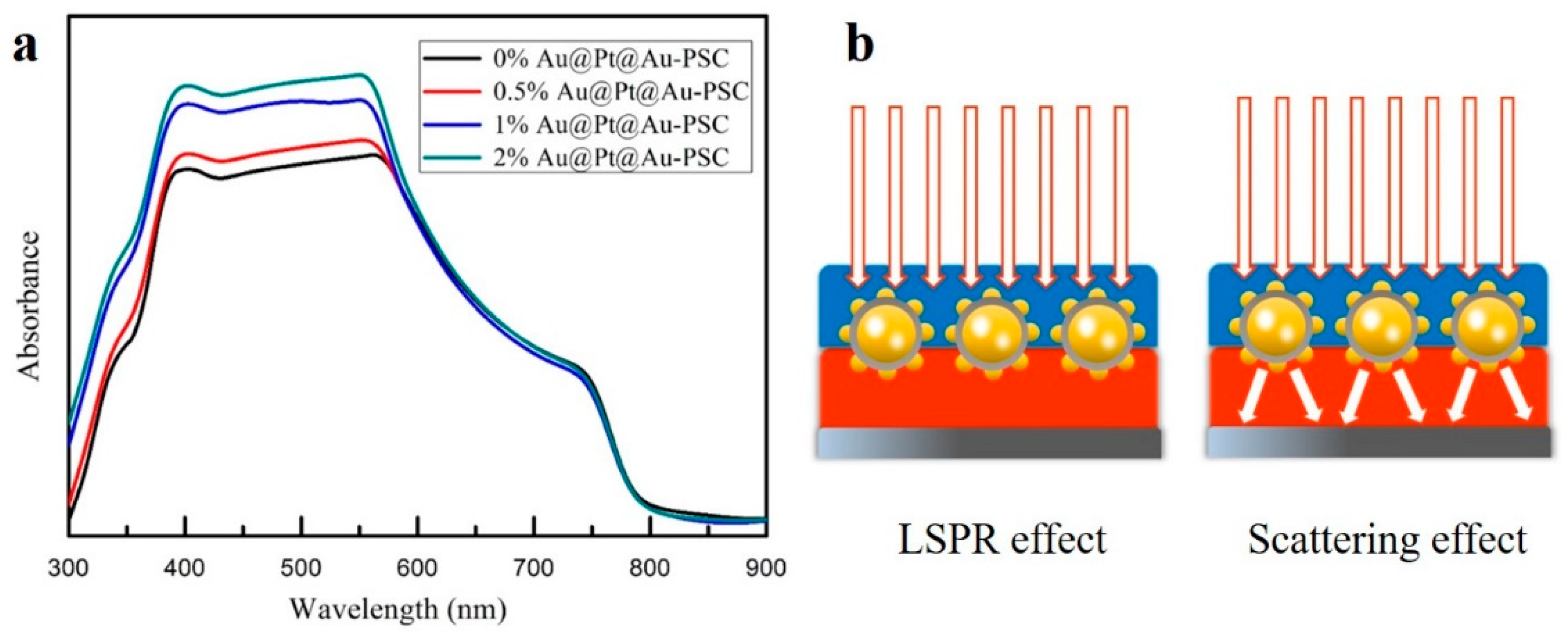

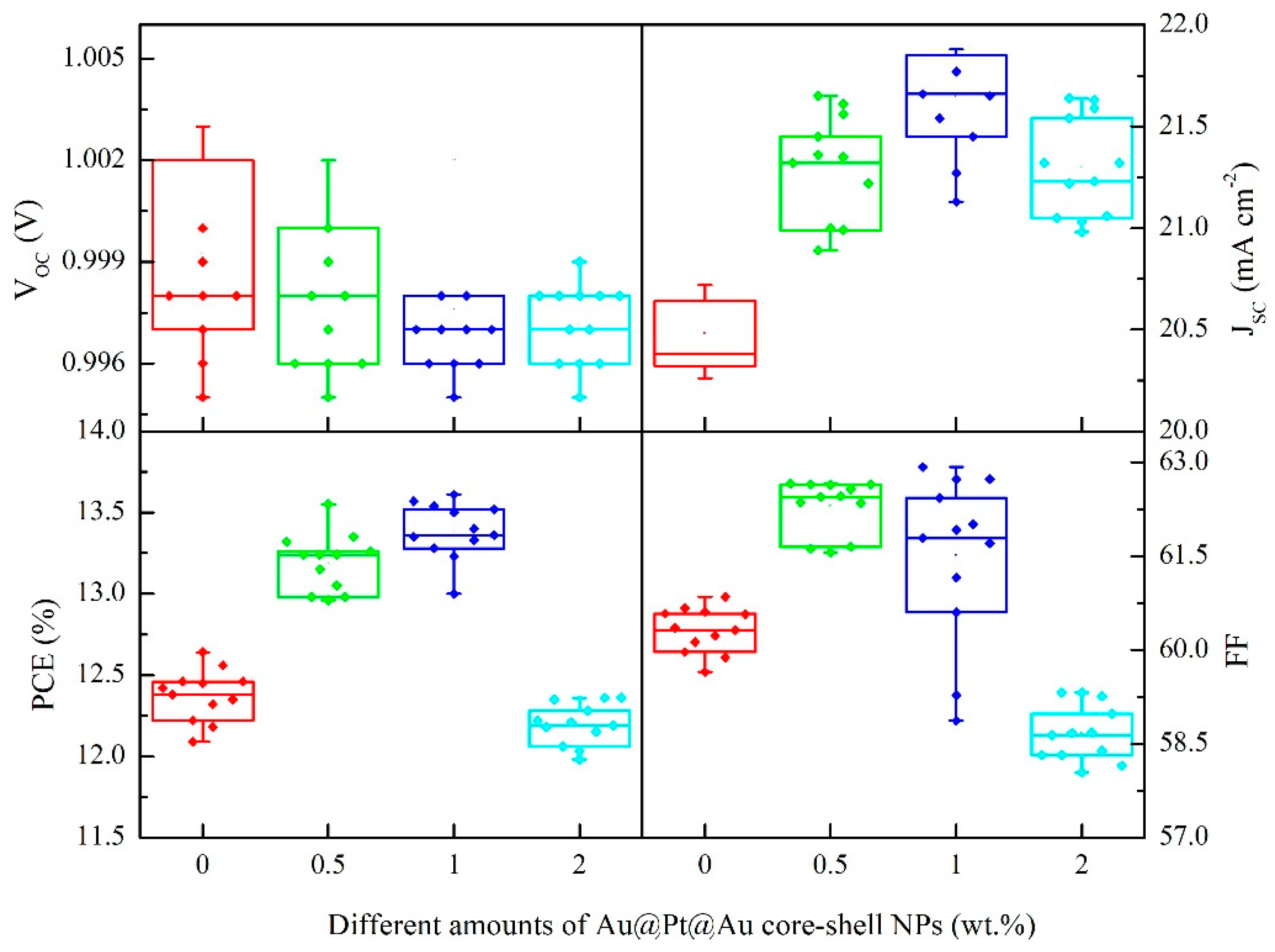

| Samples | VOC (V) | JSC (mA∙cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 0 wt.% Au@Pt@Au-TiO2 | 1.00 ± 0.01 | 20.5 ± 0.2 | 60.3 ± 0.4 | 12.4 ± 0.2 |
| 0.5 wt.% Au@Pt@Au-TiO2 | 1.00 ± 0.01 | 21.3 ± 0.2 | 62.3 ± 0.4 | 13.2 ± 0.2 |
| 1 wt.% Au@Pt@Au-TiO2 | 1.00 ± 0.01 | 21.6 ± 0.3 | 61.5 ± 1.3 | 13.4 ± 0.2 |
| 2 wt.% Au@Pt@Au-TiO2 | 1.00 ± 0.01 | 21.3 ± 0.2 | 58.7 ± 0.5 | 12.2 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhu, X.; Li, S.; Chen, M.; Liu, N.; Yang, H.; Ran, M.; Lu, H.; Yang, Y. Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles. Nanomaterials 2019, 9, 1263. https://doi.org/10.3390/nano9091263
Wang B, Zhu X, Li S, Chen M, Liu N, Yang H, Ran M, Lu H, Yang Y. Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles. Nanomaterials. 2019; 9(9):1263. https://doi.org/10.3390/nano9091263
Chicago/Turabian StyleWang, Bao, Xiangyu Zhu, Shuhan Li, Mengwei Chen, Nan Liu, Hao Yang, Meiqing Ran, Haifei Lu, and Yingping Yang. 2019. "Enhancing the Photovoltaic Performance of Perovskite Solar Cells Using Plasmonic Au@Pt@Au Core-Shell Nanoparticles" Nanomaterials 9, no. 9: 1263. https://doi.org/10.3390/nano9091263





