Characteristics of p-Type Conduction in P-Doped MoS2 by Phosphorous Pentoxide during Chemical Vapor Deposition
Abstract
:1. Introduction
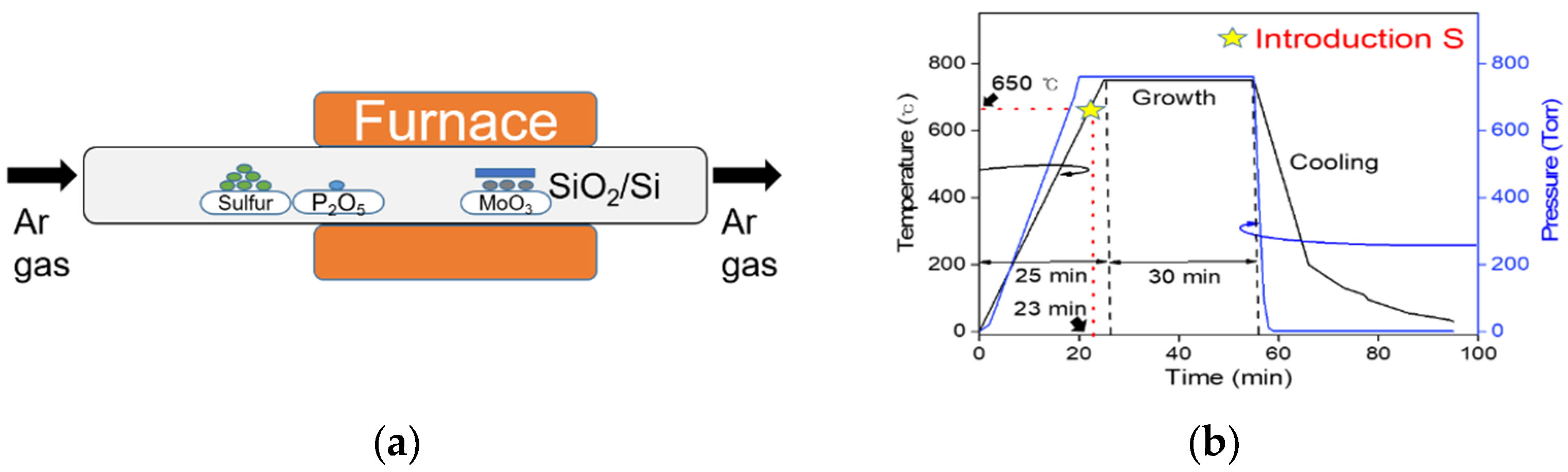
2. Experimental
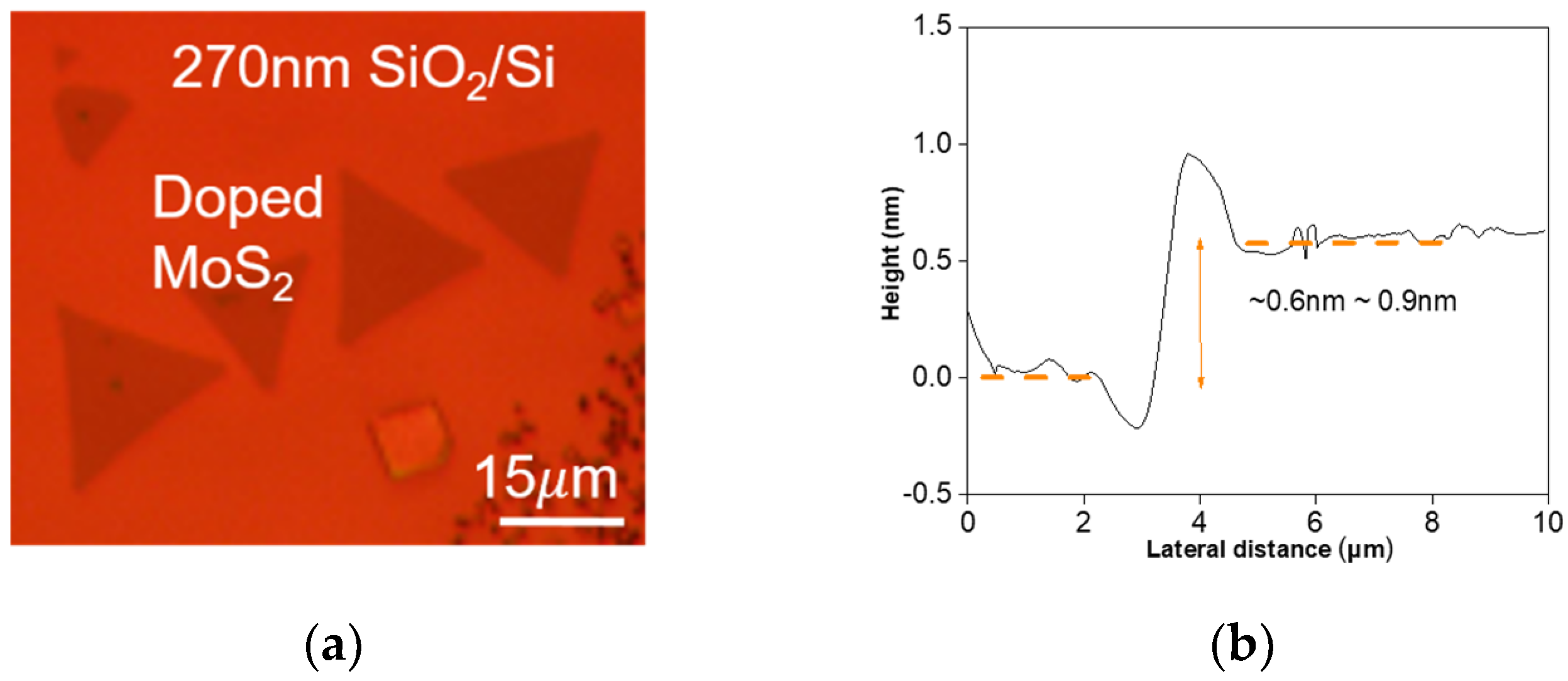
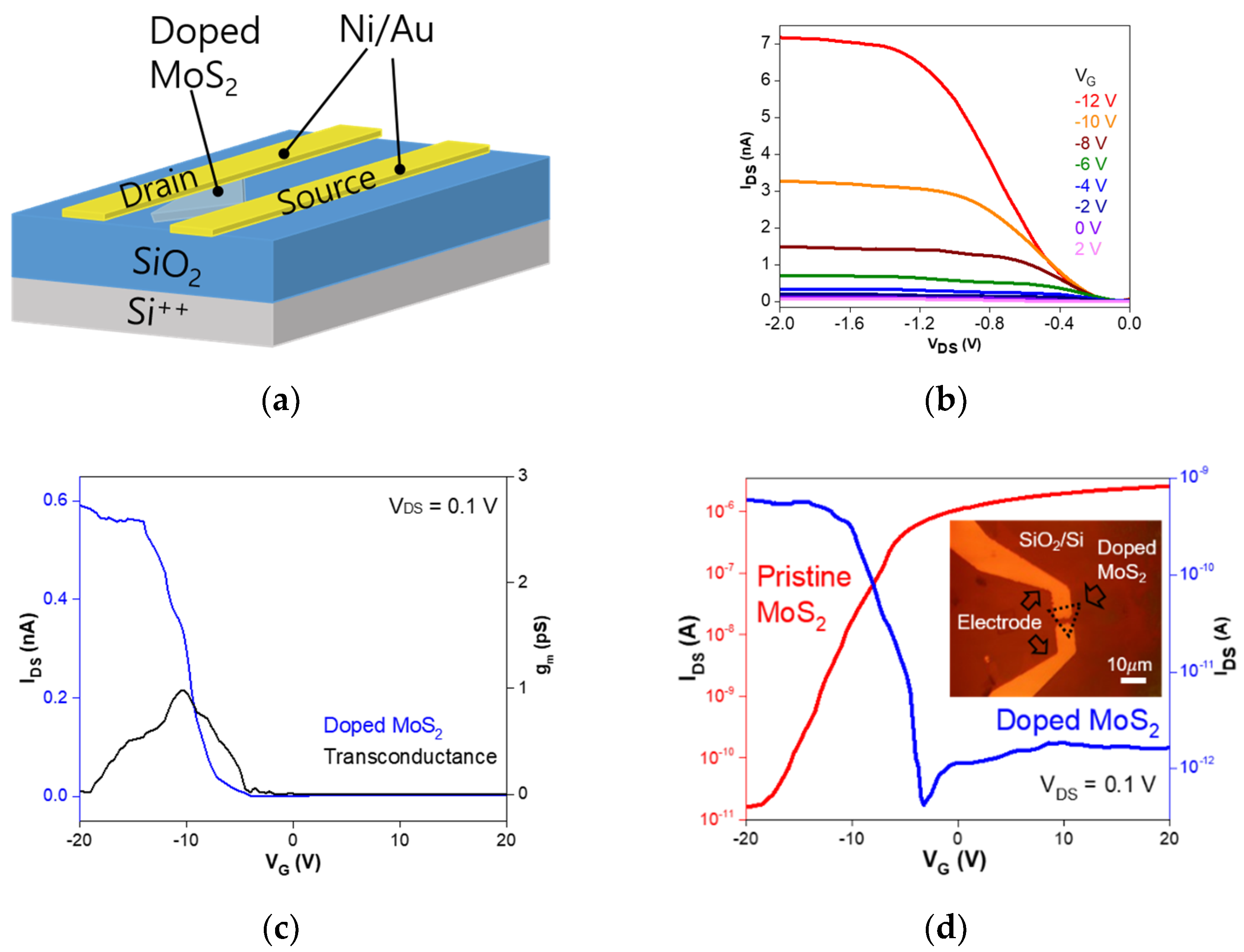
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Griorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hon, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yin, X.; Ulin-Avila, E.; Geng, B.; Zentgraf, T.; Ju, L.; Wang, F.; Zhang, X. A graphene based broadband optical modulator. Nature 2011, 474, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ozlem, S.; Akkaya, U. Graphene electronics: Thinking outside the silicon box. Nat. Nanotechnol. 2009, 131, 48–49. [Google Scholar]
- Osada, M.; Sasaki, T. 2D inorganic nano-sheets: Two-dimensional dielectric nano-sheets: Novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 2012, 24, 210–228. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Yang, D.; Crozier, E.; Jiang, D.; Frindt, R. Structures of exfoliated single layers of WS2, MoS2, and MoSe2 in aqueous suspension. Phys. Rev. B 2002, 65, 125407. [Google Scholar] [CrossRef]
- Chu, D.; Pak, S.W.; Kim, E.K. Locally Gated SnS2/hBN Thin Film Transistors with a Broadband Photoresponse. Sci. Rep. 2018, 8, 10585–10593. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Chu, D.; Yoo, J.; Kim, E.K. Formation of transition metal dichalcogenides thin films with liquid phase exfoliation technique and photovoltaic applications. Sol. Energy Mater. Sol. Cells 2018, 184, 9–14. [Google Scholar] [CrossRef]
- Qiu, D.; Lee, D.U.; Pak, S.W.; Kim, E.K. Structural and optical properties of MoS2 layers grown by successive two-step chemical vapor deposition method. Thin Solid Films 2015, 587, 47–51. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Laskar, M.R.; Nath, D.N.; Ma, L.; Lee, E.W.; Lee, C.H.; Kent, T.; Yang, Z.; Mishra, R.; Roldan, M.A.; Idrobo, J.-C.; et al. p-type doping of MoS2 thin films using Nb. Appl. Phys. Lett. 2014, 104, 092104. [Google Scholar] [CrossRef]
- Momose, T.; Nakamura, A.; Daniel, M.; Shimomura, M. Phosphorous doped p-type MoS2 polycrystalline thin films via direct sulfurization of Mo film. AIP Adv. 2018, 8, 025009. [Google Scholar] [CrossRef]
- Dolui, K.; Rungger, I.; Pemmaraju, C.D.; Sanvito, S. Possible doping strategies for MoS2 monolayers: Anab initiostudy. Phys. Rev. B 2013, 88, 075429. [Google Scholar] [CrossRef]
- Xu, E.Z.; Liu, H.M.; Park, K.; Li, Z.; Losovyj, Y.; Starr, M.; Werbianskyj, M.; Fertig, H.A.; Zhang, S.X. p-Type transition-metal doping of large-area MoS2 thin films grown by chemical vapor deposition. Nanoscale 2017, 9, 3576–3584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Bersch, B.M.; Joshi, J.; Addou, R.; Cormier, C.R.; Zhang, C.; Xu, K.; Briggs, N.C.; Wang, K.; Subramanian, S.; et al. Tuning the Electronic and Photonic Properties of Monolayer MoS2 via In Situ Rhenium Substitutional Doping. Adv. Funct. Mater. 2018, 28, 1706950. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, S.; Wang, J.; Azcatl, A.; Lu, N.; Addou, R.; Wang, N.; Zhou, C.; Lerach, J.; Bojan, V.; et al. Manganese Doping of Monolayer MoS2: The Substrate Is Critical. Nano Lett. 2015, 15, 6586–6591. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Zhan, Y.; Zhang, P.; Wu, R.; Jiang, T.; Wu, S.; Wang, H.; Zhao, Y.; Nan, T.; et al. Controllable growth of monolayer MoS2 by chemical vapor deposition via close MoO2 precursor for electrical and optical applications. Nanotechnology 2017, 28, 084001. [Google Scholar] [CrossRef]
- Wang, S.; Rong, Y.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape Evolution of Monolayer MoS2 Crystals Grown by Chemical Vapor Deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Lee, C.G.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single-and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Azcatl, A.; Qin, X.; Prakash, A.; Zhang, C.; Cheng, L.; Wang, Q.; Lu, N.; Kim, M.J.; Kim, J.; Cho, K.; et al. Covalent Nitrogen Doping and Compressive Strain in MoS2 by Remote N2 Plasma Exposure. Nano Lett. 2016, 16, 5437–5443. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Bera, A.; Muthu, D.V.S.; Bhowmick, S.; Waghmare, U.V.; Sood, A.K. Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys. Rev. B 2012, 85, 161403. [Google Scholar] [CrossRef]
- Kukucska, G.; Koltai, J. Theoretical Investigation of Strain and Doping on the Raman Spectra of Monolayer MoS2. Phys. Status Solidi (B) 2017, 254, 1700184. [Google Scholar] [CrossRef]
- Suh, J.; Park, T.E.; Lin, D.Y.; Fu, D.; Park, J.; Jung, H.J.; Chen, Y.; Ko, C.; Jang, C.; Sun, Y.; et al. Doping against the native propensity of MoS2: Degenerate hole doping by cation substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Velicky, M.; Bissett, M.A.; Woods, C.R.; Toth, P.S.; Georgiou, T.; Kinloch, I.A.; Novoselov, K.S.; Dryfe, R.A. Photoelectrochemistry of Pristine Mono- and Few-Layer MoS2. Nano Lett. 2016, 16, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.; Addou, R.; Buie, C.; Wallace, R.M.; Hinkle, C.L. Defect-Dominated Doping and Contact Resistance in MoS2. ACS Nano 2014, 8, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Wang, H.; Xie, H.; Liu, R.; Zhang, S.L.; Qiu, Z.J. Thickness Considerations of Two-Dimensional Layered Semiconductors for Transistor Applications. Sci. Rep. 2016, 6, 29615–29622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hill, H.M.; Moudgil, K.; Richter, C.A.; Walker, A.R.H.; Barlow, S.; Marder, S.R.; Hacker, C.A.; Pookpanratana, S.J. Controllable, Wide-Ranging n-Doping and p-Doping of Monolayer Group 6 Transition-Metal Disulfides and Diselenides. Adv. Mater. 2018, 30, 1802991–1802999. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Park, C.-S.; Kim, T.Y.; Kim, Y.S.; Kim, E.K. Characteristics of p-Type Conduction in P-Doped MoS2 by Phosphorous Pentoxide during Chemical Vapor Deposition. Nanomaterials 2019, 9, 1278. https://doi.org/10.3390/nano9091278
Lee JS, Park C-S, Kim TY, Kim YS, Kim EK. Characteristics of p-Type Conduction in P-Doped MoS2 by Phosphorous Pentoxide during Chemical Vapor Deposition. Nanomaterials. 2019; 9(9):1278. https://doi.org/10.3390/nano9091278
Chicago/Turabian StyleLee, Jae Sang, Chang-Soo Park, Tae Young Kim, Yoon Sok Kim, and Eun Kyu Kim. 2019. "Characteristics of p-Type Conduction in P-Doped MoS2 by Phosphorous Pentoxide during Chemical Vapor Deposition" Nanomaterials 9, no. 9: 1278. https://doi.org/10.3390/nano9091278





