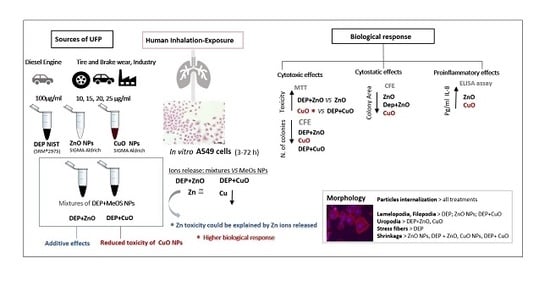
Mixture Effects of Diesel Exhaust and Metal Oxide Nanoparticles in Human Lung A549 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Particle Suspensions
2.2. Physico-Chemical Characterization of the Particle Suspensions
2.2.1. Transmission Electron Microscopy (TEM)
2.2.2. Dynamic Light Scattering (DLS)
2.2.3. Inductively Coupled Plasma-Optic Emission Spectroscopy (ICP-OES)
2.3. Cell Culture and Treatments
2.4. Biological Responses: Cytotoxicity Assays
2.4.1. Cell Viability: MTT Assay
2.4.2. Colony Forming Efficiency Assay
2.5. Quantification of Cytokine IL-8 Release
2.6. Morphological Analysis
2.6.1. Fluorescence Microscopy
2.6.2. Transmission Electron Microscopy
2.7. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticle (NP) Mixtures
3.2. Cytotoxic Effects
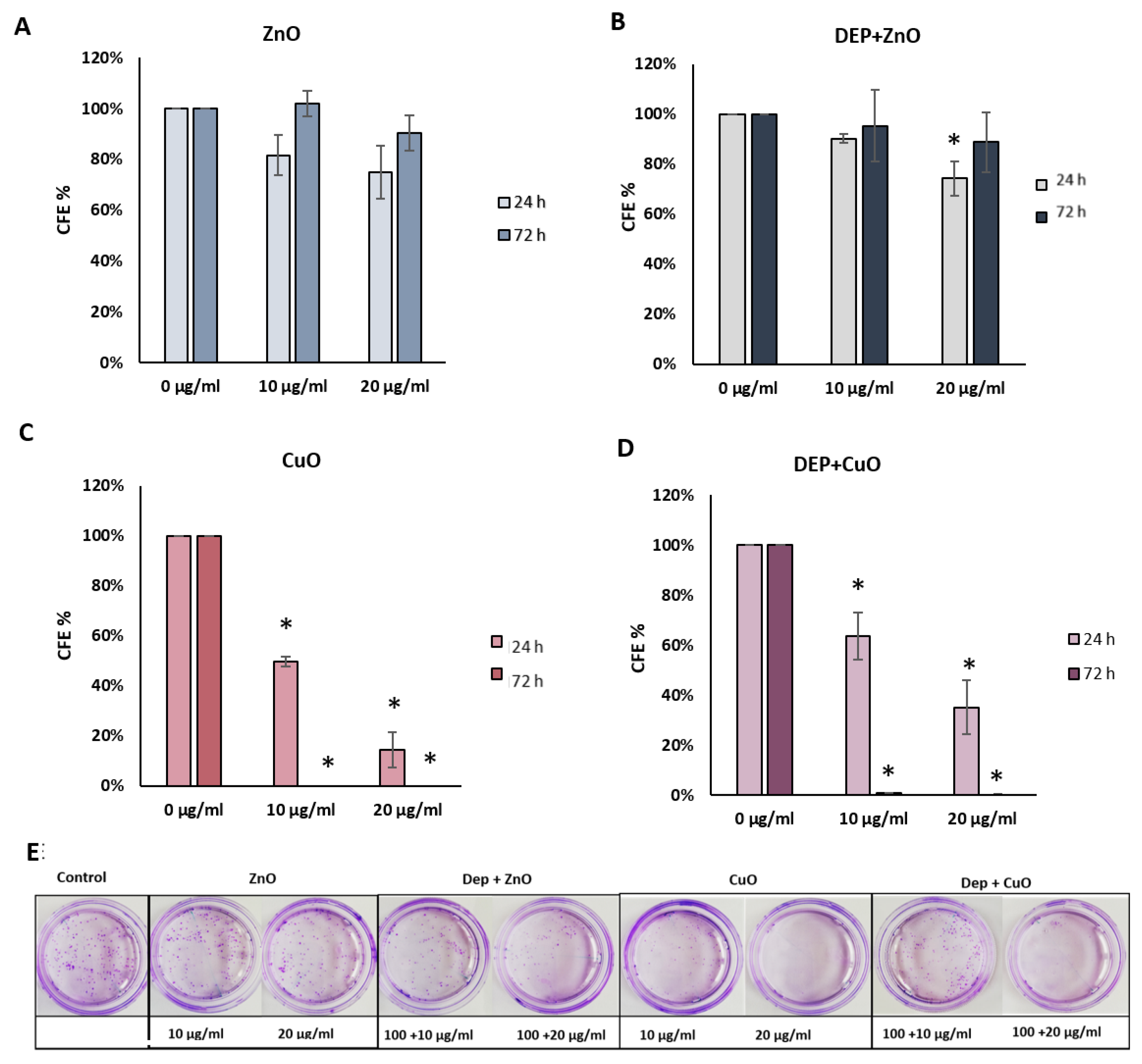
3.3. Cytostatic Effects
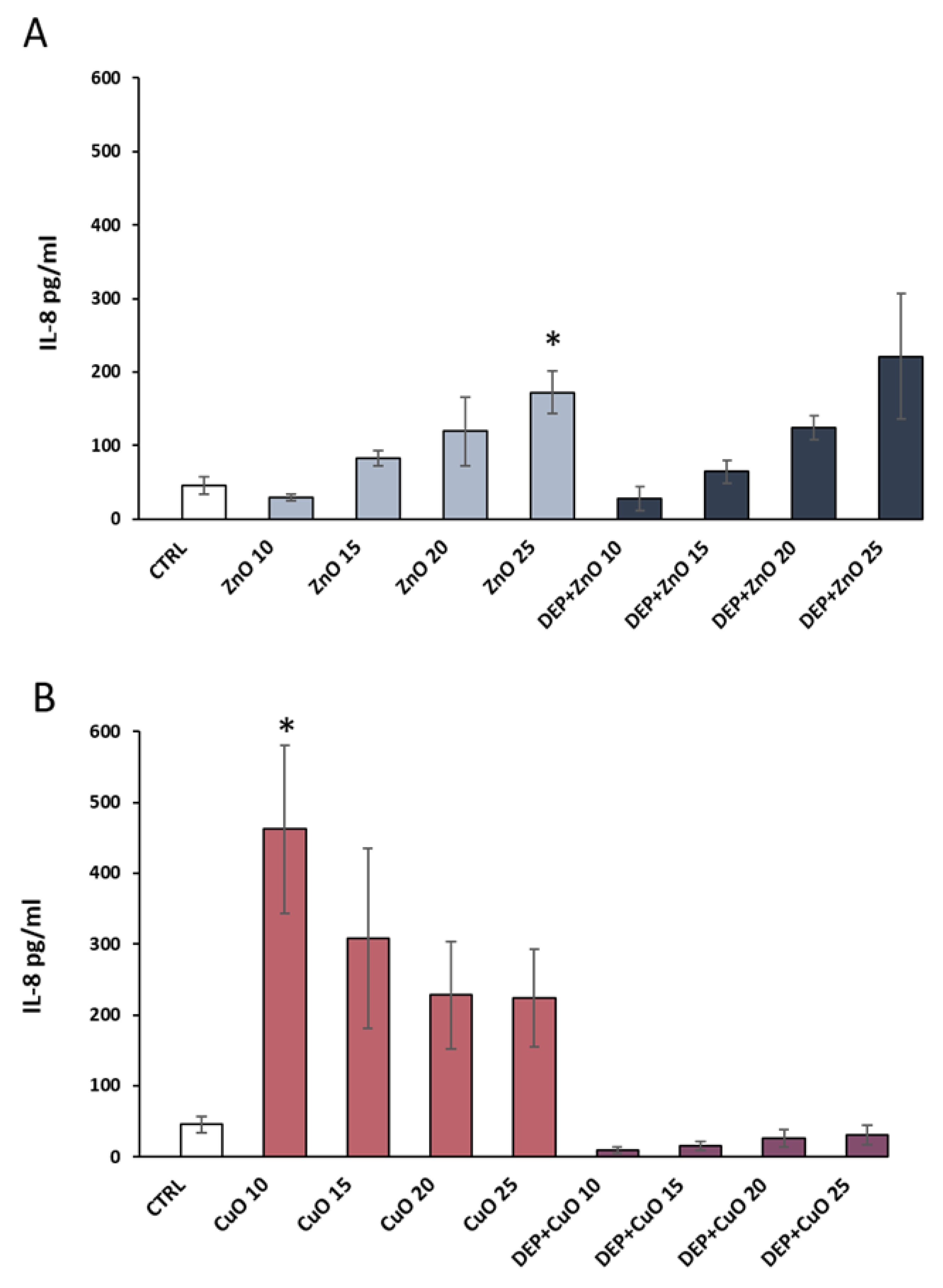
3.4. Pro-Inflammatory Effects
3.5. Cells Morphology
3.5.1. Electron Microscopy
3.5.2. Fluorescence Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pope, C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung Cancer, Cardiopulmonary Mortality, and Long-term Exposure to Fine Particulate Air Pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, W.; Felten, K.; Sommerer, K.; Scheuch, G.; Meyer, G.; Meyer, P.; Häussinger, K.; Kreyling, W.G. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008, 177, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Ott, W. Personal exposure to ultrafine particles. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.-E. Diesel Exhaust Particles. Inhal. Toxicol. 2007, 19 (Suppl. S1), 241–244. [Google Scholar] [CrossRef] [PubMed]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Santo, N.; Fascio, U.; Moschini, E.; Freddi, S.; Chirico, G.; Camatini, M.; Mantecca, P. Nano-sized CuO, TiO2 and ZnO affect Xenopus laevis development. Nanotoxicology 2012, 6, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Abu-Allaban, M.; Gillies, J.A.; Gertler, A.W.; Clayton, R.; Proffitt, D. Tailpipe, resuspended road dust, and brake-wear emission factors from on-road vehicles. Atmos. Environ. 2003, 37, 5283–5293. [Google Scholar] [CrossRef]
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. 2015, 22, 2491–2504. [Google Scholar] [CrossRef]
- Gasser, M.; Riediker, M.; Mueller, L.; Perrenoud, A.; Blank, F.; Gehr, P.; Rothen-Rutishauser, B. Toxic effects of brake wear particles on epithelial lung cells in vitro. Part. Fibre Toxicol. 2009, 6, 30. [Google Scholar] [CrossRef]
- Hjortenkrans, D.S.T.; Bergbäck, B.G.; Häggerud, A.V. Metal emissions from brake linings and tires: Case studies of Stockholm, Sweden 1995/1998 and 2005. Environ. Sci. Technol. 2007, 41, 5224–5230. [Google Scholar] [CrossRef]
- Harrison, R.M.; Jones, A.M.; Gietl, J.; Yin, J.; Green, D.C. Estimation of the contributions of brake dust, tire wear, and resuspension to nonexhaust traffic particles derived from atmospheric measurements. Environ. Sci. Technol. 2012, 46, 6523–6529. [Google Scholar] [CrossRef] [PubMed]
- Samal, S. High-Temperature Oxidation of Metals. In High Temperature Corrosion; InTechOpen: London, UK, 2016. [Google Scholar]
- Walkowicz, M.; Osuch, P.; Smyrak, B.; Knych, T.; Rudnik, E.; Cieniek, Ł.; Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Bulanda, M. Impact of oxidation of copper and its alloys in laboratory-simulated conditions on their antimicrobial efficiency. Corros. Sci. 2018, 140, 321–332. [Google Scholar] [CrossRef]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.; Donaldson, K.; Duffin, R.; Tran, L.; Kelly, F.; Mudway, I.; Morin, J.P.; Guest, R.; Jenkinson, P.; Samaras, Z.; et al. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive—A case study. Inhal. Toxicol. 2008, 20, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitr. 2009, 23, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamed, M.; Siddiqui, M.A.; Akhtar, M.J.; Ahmad, I.; Pant, A.B.; Alhadlaq, H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef]
- Moschini, E.; Gualtieri, M.; Colombo, M.; Fascio, U.; Camatini, M.; Mantecca, P. The modality of cell–particle interactions drives the toxicity of nanosized CuO and TiO2 in human alveolar epithelial cells. Toxicol. Lett. 2013, 222, 102–116. [Google Scholar] [CrossRef]
- Buerki-Thurnherr, T.; Xiao, L.; Diener, L.; Arslan, O.; Hirsch, C.; Maeder-Althaus, X.; Grieder, K.; Wampfler, B.; Mathur, S.; Wick, P.; et al. In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology 2013, 7, 402–416. [Google Scholar] [CrossRef]
- Schwarze, P.E.; Totlandsdal, A.I.; Låg, M.; Refsnes, M.; Holme, J.A.; Øvrevik, J. Inflammation-related effects of diesel engine exhaust particles: Studies on lung cells in vitro. Biomed. Res. Int. 2013, 2013, 685142. [Google Scholar] [CrossRef]
- Stenfors, N.; Nordenhäll, C.; Salvi, S.S.; Mudway, I.; Söderberg, M.; Blomberg, A.; Helleday, R.; Levin, J.O.; Holgate, S.T.; Kelly, F.J.; et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur. Respir. J. 2004, 23, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douki, T.; Corbière, C.; Preterre, D.; Martin, P.J.; Lecureur, V.; André, V.; Landkocz, Y.; Pottier, I.; Keravec, V.; Fardel, O.; et al. Comparative study of diesel and biodiesel exhausts on lung oxidative stress and genotoxicity in rats. Environ. Pollut. 2018, 235, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Zerboni, A.; Marchetti, S.; Longhin, E.; Priola, M.; Camatini, M.; Mantecca, P. In vitro pulmonary and vascular effects induced by different diesel exhaust particles. Toxicol. Lett. 2019, 306, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Donato, M.T.; Gómez-Lechón, M.J. Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp. Toxicol. Pathol. 2005, 57 (Suppl. S1), 189–204. [Google Scholar] [CrossRef]
- Fröhlich, E.; Salar-Behzadi, S. Toxicological Assessment of Inhaled Nanoparticles: Role of in Vivo, ex Vivo, in Vitro, and in Silico Studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef]
- Gottardo, S.; Alessandrelli, M.; Amenta, V.; Atluri, R.; Barberio, G.; Bekker, C.; Bergonzo, P.; Bleeker, E.; Booth, A.; Borges, T.; et al. NANoREG Framework for the Safety Assessment of Nanomaterials; European Commission Joint Research Centre: Ispra, Italy, 2017. [Google Scholar] [CrossRef]
- Van Teunenbroek, T. NANoREG, a Common European Approach to the Regulatory Testing of Nanomaterials; Final Report (part 1); European Commission Joint Research Centre: Ispra, Italy, 2016. [Google Scholar]
- Longhin, E.; Gualtieri, M.; Capasso, L.; Bengalli, R.; Mollerup, S.; Holme, J.A.; Øvrevik, J.; Casadei, S.; Di Benedetto, C.; Parenti, P.; et al. Physico-chemical properties and biological effects of diesel and biomass particles. Environ. Pollut. 2016, 215, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Ponti, J.; Kinsner-Ovaskainen, A.; Norlén, H.; Altmeyer, S.; Andreoli, C.; Chevillard, S.; De Angelis, I.; Chung, S.-T.; Eom, I.; Fujita, K.; et al. Interlaboratory Comparison Study of the Colony Forming Efficiency Assay for Assessing Cytotoxicity of Nanomaterials; Publications Office of the European Union: Ispra, Italy, 2014. [Google Scholar] [CrossRef]
- Bengalli, R.; Gualtieri, M.; Capasso, L.; Urani, C.; Camatini, M. Impact of zinc oxide nanoparticles on an in vitro model of the human air-blood barrier. Toxicol. Lett. 2017, 279, 22–32. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Arul, V.; Selvan, M.; Anand, R.B.; Udayakumar, M. Effects of cerium oxide nanoparticle addition in diesel and diesel-biodiesel-ethanol blends on the performance and emission characteristics of a CI engine. J. Eng. Appl. Sci. 2009, 4, 1819–6608. [Google Scholar]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Asweto, C.; Wu, J.; Hu, H.; Feng, L.; Yang, X.; Duan, J.; Sun, Z. Combined Effect of Silica Nanoparticles and Benzo[a]pyrene on Cell Cycle Arrest Induction and Apoptosis in Human Umbilical Vein Endothelial Cells. Int. J. Environ. Res. Public Health 2017, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Zhang, M.; Dittmar, M.; Lulla, A.; Araujo, J.A. Heme oxygenase-1 protects endothelial cells from the toxicity of air pollutant chemicals. Toxicol. Appl. Pharmacol. 2015, 284, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.Y.; Wang, J.S.; Chao, M.W. Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation. Cardiovasc. Toxicol. 2017, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.L.; Goth-Goldstein, R.; Lucas, D.; Koshland, C.P. Particle-induced artifacts in the MTT and LDH viability assays. Chem. Res. Toxicol. 2012, 25, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zebda, R.; Drake, S.J.; Sayes, C.M. Synergistic effect of co-exposure to carbon black and Fe2O3 nanoparticles on oxidative stress in cultured lung epithelial cells. Part Fibre Toxicol. 2009, 6, 4. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, J.; Li, Y.; Yu, Y.; Jin, M.; Li, C.; Wang, Y.; Sun, Z. Combined toxicity of amorphous silica nanoparticles and methylmercury to human lung epithelial cells. Ecotoxicol. Environ. Saf. 2015, 112, 144–152. [Google Scholar] [CrossRef]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef]
- Wongrakpanich, A.; Geary, S.M.; Morris, A.S.; Salem, A.K.; Mudunkotuwa, I.A.; Grassian, V.H.; Mapuskar, K.A.; Spitz, D.R. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar] [CrossRef]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, W.S.; Duffin, R.; Howie, S.E.M.; Scotton, C.J.; Wallace, W.A.H.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+dissolution inside lysosomes. Part Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Foucaud, L.; Barlow, P.G.; Hutchison, G.R.; Sales, J.; Simpson, R.J.; Stone, V. Nanoparticle interactions with zinc and iron: Implications for toxicology and inflammation. Toxicol. Appl. Pharmacol. 2007, 225, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Uski, O.; Jalava, P.I.; Happo, M.S.; Torvela, T.; Leskinen, J.; Mäki-Paakkanen, J.; Tissari, J.; Sippula, O.; Lamberg, H.; Jokiniemi, J.; et al. Effect of fuel zinc content on toxicological responses of particulate matter from pellet combustion in vitro. Sci. Total Environ. 2015, 511, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, S.; Longhin, E.; Bengalli, R.; Avino, P.; Stabile, L.; Buonanno, G.; Colombo, A.; Camatini, M.; Mantecca, P. In vitro lung toxicity of indoor PM10 from a stove fueled with different biomasses. Sci. Total Environ. 2019, 649, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Mantecca, P.; Kasemets, K.; Deokar, A.; Perelshtein, I.; Gedanken, A.; Bahk, Y.K.; Kianfar, B.; Wang, J. Airborne Nanoparticle Release and Toxicological Risk from Metal-Oxide-Coated Textiles: Toward a Multiscale Safe-by-Design Approach. Environ. Sci. Technol. 2017, 51, 9305–9317. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.; Holme, J.A.; Gualtieri, M.; Camatini, M.; Øvrevik, J. Milan winter fine particulate matter (wPM2.5) induces IL-6 and IL-8 synthesis in human bronchial BEAS-2B cells, but specifically impairs IL-8 release. Toxicol. Vitr. 2018, 52, 365–373. [Google Scholar] [CrossRef]
- Schmoranzer, J.; Simon, S.M. Role of Microtubules in Fusion of Post-Golgi Vesicles to the Plasma Membrane. Mol. Biol. Cell 2003, 14, 1558–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ispanixtlahuatl-Meráz, O.; Schins, R.P.F.; Chirino, Y.I. Cell type specific cytoskeleton disruption induced by engineered nanoparticles. Environ. Sci. Nano 2018, 5, 228–245. [Google Scholar] [CrossRef]
- Vuong, N.Q.; Goegan, P.; Mohottalage, S.; Breznan, D.; Ariganello, M.; Williams, A.; Elisma, F.; Karthikeyan, S.; Vincent, R.; Kumarathasan, P. Proteomic changes in human lung epithelial cells (A549) in response to carbon black and titanium dioxide exposures. J. Proteom. 2016, 149, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Dornhof, R.; Maschowski, C.; Osipova, A.; Gieré, R.; Seidl, M.; Merfort, I.; Humar, M. Stress fibers, autophagy and necrosis by persistent exposure to PM2.5 from biomass combustion. PLoS ONE 2017, 12, e0180291. [Google Scholar] [CrossRef]
- Kenzaoui, B.H.; Bernasconi, C.C.; Guney-Ayra, S.; Juillerat-Jeanneret, L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem. J. 2012, 441, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO Nanoparticle Interaction with Human Epithelial Cells: Cellular Uptake, Location, Export, and Genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Karg, E.; Brendel, E.; Hinze-Heyn, H.; Maier, K.L.; Eickelberg, O.; Stoeger, T.; Schmid, O. Inflammatory and Oxidative Stress Responses of an Alveolar Epithelial Cell Line to Airborne Zinc Oxide Nanoparticles at the Air-Liquid Interface: A Comparison with Conventional, Submerged Cell-Culture Conditions. Biomed. Res. Int. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Fizeșan, I.; Cambier, S.; Moschini, E.; Chary, A.; Nelissen, I.; Ziebel, J.; Audinot, J.-N.; Wirtz, T.; Kruszewski, M.; Pop, A.; et al. In vitro exposure of a 3D-tetraculture representative for the alveolar barrier at the air-liquid interface to silver particles and nanowires. Part Fibre Toxicol. 2019, 16, 14. [Google Scholar] [CrossRef]
- Loxham, M.; Morgan-Walsh, R.J.; Cooper, M.J.; Blume, C.; Swindle, E.J.; Dennison, P.W.; Howarth, P.H.; Cassee, F.R.; Teagle, D.A.H.; Palmer, M.R.; et al. The Effects on Bronchial Epithelial Mucociliary Cultures of Coarse, Fine, and Ultrafine Particulate Matter from an Underground Railway Station. Toxicol. Sci. 2015, 145, 98–107. [Google Scholar] [CrossRef]
- Klein, S.G.; Cambier, S.; Hennen, J.; Legay, S.; Serchi, T.; Nelissen, I.; Chary, A.; Moschini, E.; Krein, A.; Blömeke, B.; et al. Endothelial responses of the alveolar barrier in vitro in a dose-controlled exposure to diesel exhaust particulate matter. Part Fibre Toxicol. 2017, 14, 7. [Google Scholar] [CrossRef]
- Fizeșan, I.; Chary, A.; Cambier, S.; Moschini, E.; Serchi, T.; Nelissen, I.; Kiss, B.; Pop, A.; Loghin, F.; Gutleb, A.C. Responsiveness assessment of a 3D tetra-culture alveolar model exposed to diesel exhaust particulate matter. Toxicol. Vitr. 2018, 53, 67–79. [Google Scholar] [CrossRef]





| ZnO | DEP + ZnO | CuO | DEP + CuO | DEP (2975) | |
|---|---|---|---|---|---|
| Milli-Q Z-average ± SE (nm) | 275.7 ± 9 | 179.7 ± 2 | 208.43 ± 2 | 217.07 ± 3 | 263.03 ± 3 |
| PdI | 0.361 | 0.352 | 0.209 | 0.223 | 0.29 |
| Opti-MEM 1% FBS Z-average ± SE (nm) | 314.38 ± 204 | 207.37 ± 7 | 464.67 ± 2 | 275.7 ± 3 | 320.8 ± 6 |
| PdI | 0.63 | 0.52 | 0.35 | 0.22 | 0.21 |
| Milli-Q Z-potential ± SE (mV) | 25 ± 0.13 | −19 ± 0.21 | 12 ± 0.6 | −18 ± 0.09 | −35 ± 0.52 |
| ZnO | DEP + ZnO | CuO | DEP + CuO | |
|---|---|---|---|---|
| 3 h ppm Zn | 9.36 ± 1.1 | 8.3 ± 0.3 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 3 h % Zn | 51.2% | 51.5% | 0% | 0% |
| 24 h ppm Zn | 11.1 ± 0.1 | 10.0 ± 0.1 | nd | nd |
| 24 h % Zn | 69.4% | 62.5% | 43.3% | 26.7% |
| 3 h ppm Cu | nd | nd | 2.4 ± 0.1 | 2.4 ± 0.1 |
| 3 h % Cu | 0% | 0% | 14.7% | 14.5% |
| 24 h ppm Cu | nd | nd | 7.1 ± 0.2 | 4.4 ± 0.2 |
| 24 h % Cu | 0% | 0% | 43.3% | 26.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerboni, A.; Bengalli, R.; Baeri, G.; Fiandra, L.; Catelani, T.; Mantecca, P. Mixture Effects of Diesel Exhaust and Metal Oxide Nanoparticles in Human Lung A549 Cells. Nanomaterials 2019, 9, 1302. https://doi.org/10.3390/nano9091302
Zerboni A, Bengalli R, Baeri G, Fiandra L, Catelani T, Mantecca P. Mixture Effects of Diesel Exhaust and Metal Oxide Nanoparticles in Human Lung A549 Cells. Nanomaterials. 2019; 9(9):1302. https://doi.org/10.3390/nano9091302
Chicago/Turabian StyleZerboni, Alessandra, Rossella Bengalli, Giulia Baeri, Luisa Fiandra, Tiziano Catelani, and Paride Mantecca. 2019. "Mixture Effects of Diesel Exhaust and Metal Oxide Nanoparticles in Human Lung A549 Cells" Nanomaterials 9, no. 9: 1302. https://doi.org/10.3390/nano9091302