Mg Doped CuCrO2 as Efficient Hole Transport Layers for Organic and Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. CuCrO2 (CCO) and Mg:CCO Preparation
2.1.1. Nanoparticle Synthesis
2.1.2. Suspension Preparation
2.1.3. Film Preparation
2.2. Materials Characterizaton
2.3. Solar Cell Fabrication and Testing
2.3.1. P3HT:PC61BM OSCs
2.3.2. PFBT2Se2Th:PC71BM OSCs
2.3.3. PTB7-Th:ITIC OSCs
2.3.4. MAPbI3 PSCs
3. Results and Discussion
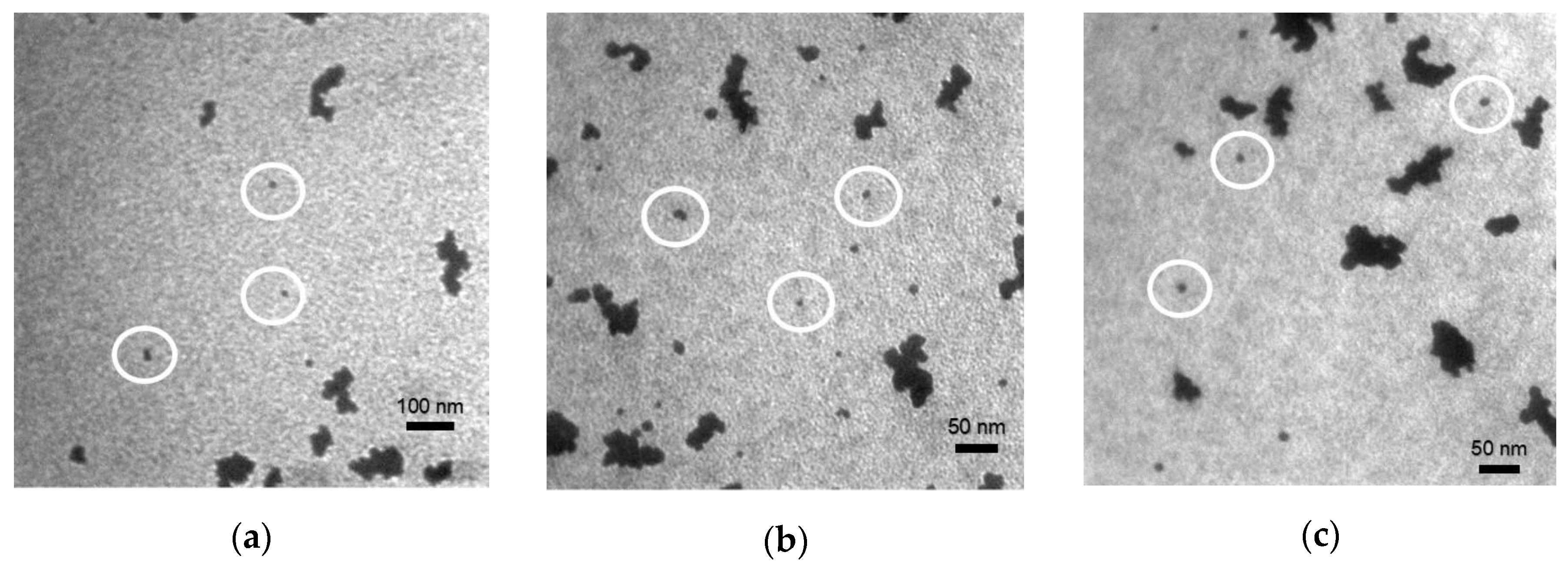
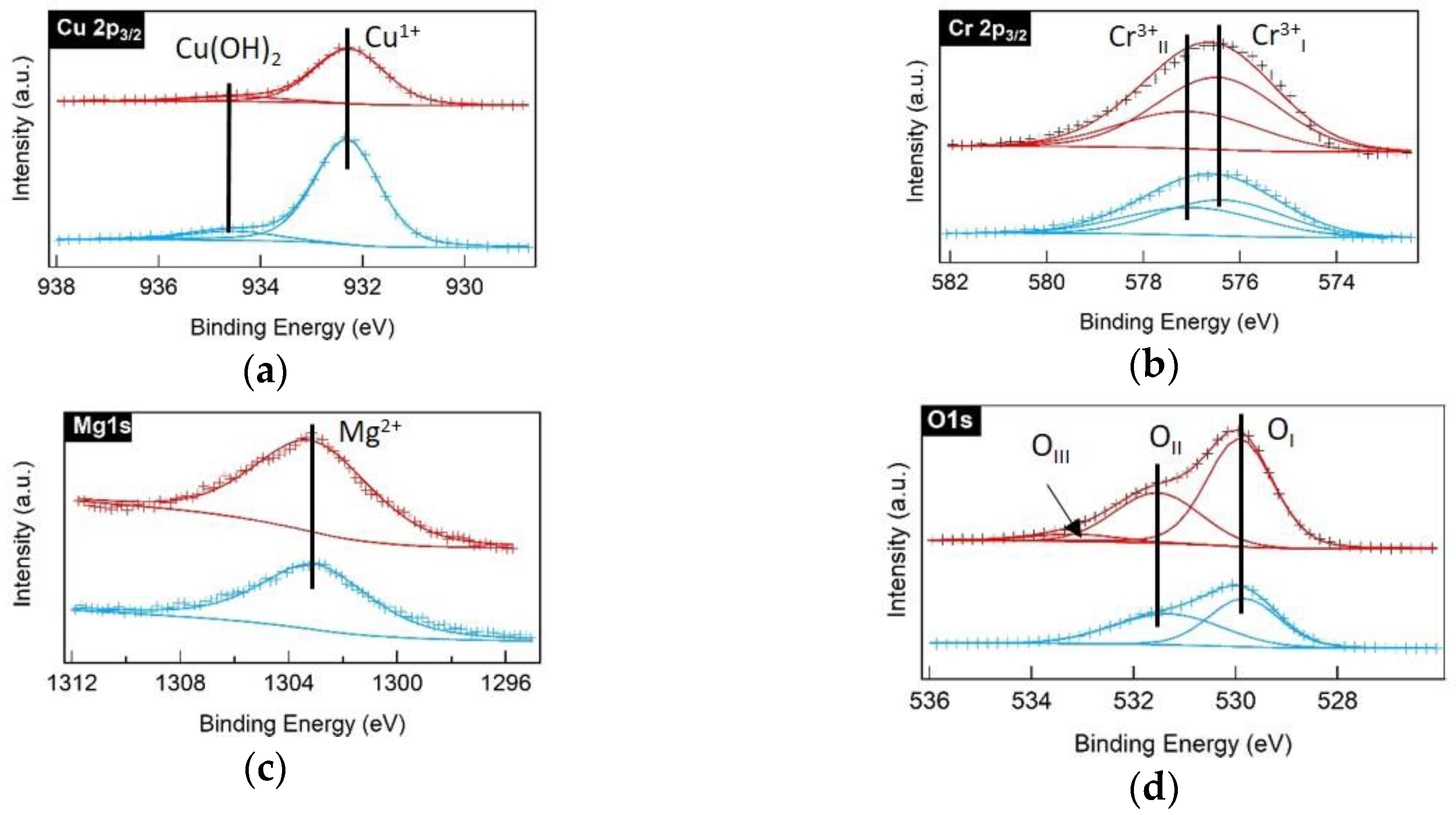
3.1. Structural, Compositional, and Morphological Characterizations
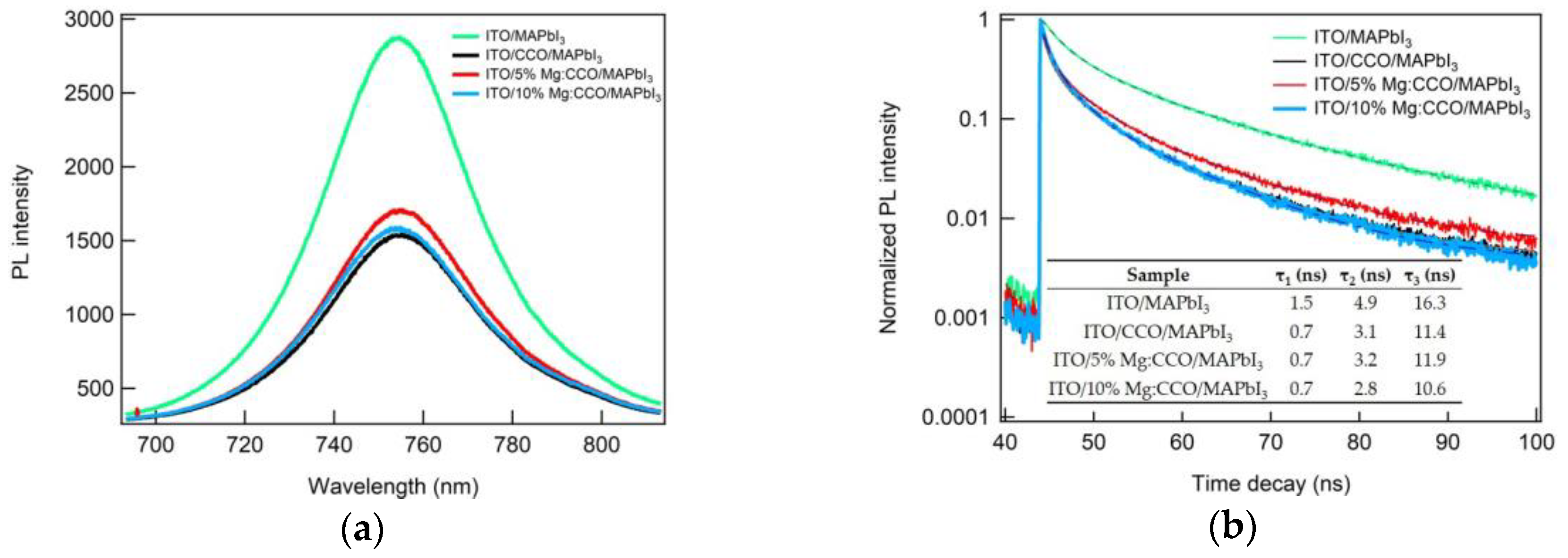
3.2. Optical and Electronic Characterizations
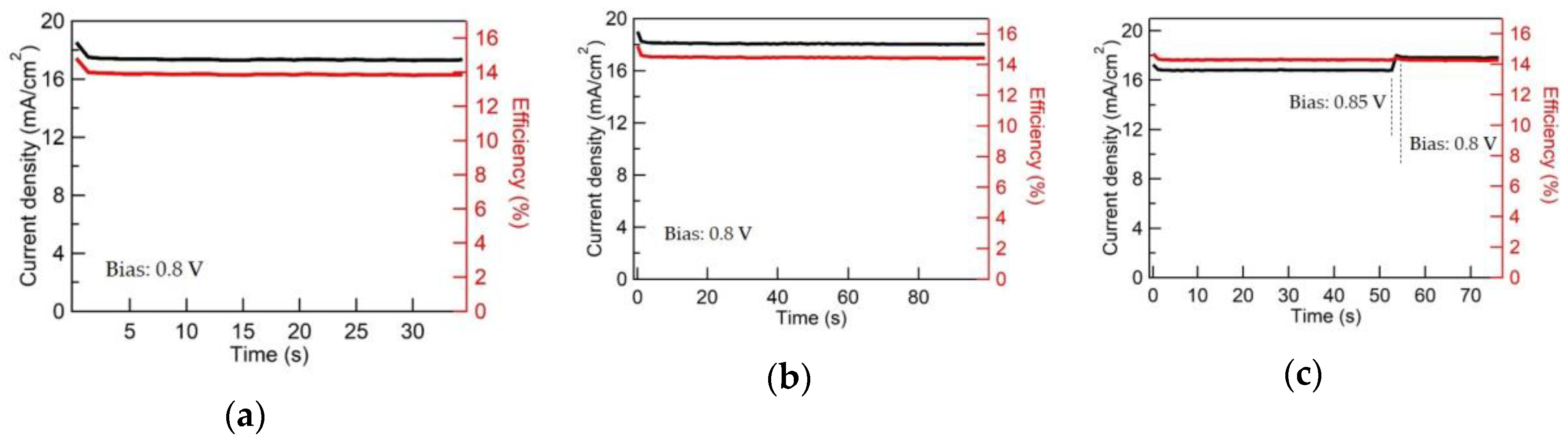
3.3. CCO and Mg:CCO as HTLs in OSCs and PSCs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Hany, R.; Lin, H.; Castro, F.A. Focus issue on organic and hybrid photovoltaics. Sci. Technol. Adv. Mater. 2019, 20, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.-L.; Jen, A.K.-Y. Recent advances in solution-processed interfacial materials for efficient and stable polymer solar cells. Energy Environ. Sci. 2012, 5, 5994–6011. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.-Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ludin, N.A.; Sopian, K.; Teridi, M.A.M. A review of organic small molecule-based hole-transporting materials for meso-structured organic–inorganic perovskite solar cells. J. Mater. Chem. A 2016, 4, 15788–15822. [Google Scholar] [CrossRef]
- Su, Y.W.; Lan, S.C.; Wei, K.H. Organic photovoltaics. Mater. Today 2012, 15, 554–562. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Kawano, K.; Pacios, R.; Poplavskyy, D.; Nelson, J.; Durrant, J.R. Degradation of organic solar cells due to air exposure. Sol. Energy Mater. Sol. Cells 2006, 90, 3520–3530. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y.; Chang, W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Stubhan, T.; Li, N.; Luechinger, N.A.; Halim, S.C.; Matt, G.J.; Brabec, C.J. High Fill Factor Polymer Solar Cells Incorporating a Low Temperature Solution Processed WO3 Hole Extraction Layer. Adv. Energy Mater. 2012, 2, 1433–1438. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Yi, J.; Gao, G.F.; Koerner, H.; Park, K.; Wang, J.; Luo, K.; Vaia, R.A.; Hsu, J.W.P. Low-temperature solution-processed molybdenum oxide nanoparticle hole transport layers for organic photovoltaic devices. Adv. Energy Mater. 2012, 2, 1193–1197. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Lee, Y.-J.; Villa, M.D.A.; Malko, A.V.; Hsu, J.W.P. Effects of Contact-Induced Doping on the Behaviors of Organic Photovoltaic Devices. Nano Lett. 2015, 15, 7627–7632. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.R.; Meyer, J.; Widjonarko, N.E.; Ndione, P.F.; Sigdel, A.K.; Miedaner, A.; Lloyd, M.T.; Kahn, A.; Ginley, D.S.; Berry, J.J.; et al. Low-temperature, solution-processed molybdenum oxide hole-collection layer for organic photovoltaics. J. Mater. Chem. 2012, 22, 3249–3254. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Bhattacharya, B.; Rhee, H.-W. Review of current progress in inorganic hole-transport materials for perovskite solar cells. Appl. Mater. Today 2019, 14, 175–200. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.H.; Li, X.; Cheng, J. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, S. Electronic structure of NiO and related 3d-transition-metal compounds. Adv. Phys. 1994, 43, 183–356. [Google Scholar] [CrossRef]
- Renaud, A.; Chavillon, B.; Pleux, L.L.; Pellegrin, Y.; Blart, E.; Boujtita, M.; Pauporté, T.; Cario, L.; Jobic, S.; Odobel, F. CuGaO2: A promising alternative for NiO in p-type dye solar cells. J. Mater. Chem. 2012, 22, 14353–14356. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Sheets, W.C.; Mugnier, E.; Barnabe´, A.; Marks, T.J.; Poeppelmeier, K.R. Hydrothermal Synthesis of Delafossite-Type Oxides. Chem. Mater. 2006, 18, 7–20. [Google Scholar] [CrossRef]
- Marquardt, M.A.; Ashmore, N.A.; Cann, D.P. Crystal chemistry and electrical properties of the delafossite structure. Thin Solid Films 2006, 496, 146–156. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W. Understanding the p-type defect chemistry of CuCrO2. J. Mater. Chem. 2011, 21, 3655–3663. [Google Scholar] [CrossRef]
- Arnold, T.; Payne, D.J.; Bourlange, A.; Hu, J.P.; Egdell, R.G.; Piper, L.F.J.; Colakerol, A.D.M.; Glans, P.A.; Learmonth, T.; Smith, K.E.; et al. X-ray spectroscopic study of the electronic structure of. Phys. Rev. B 2009, 79, 075102. [Google Scholar] [CrossRef]
- Wang, J.; Lee, Y.-J.; Hsu, J.W.P. Sub-10 nm copper chromium oxide nanocrystals as a solution processed p-type hole transport layer for organic photovoltaics. J. Mater. Chem. C 2016, 4, 3607–3613. [Google Scholar] [CrossRef]
- Xiong, D.; Xu, Z.; Zeng, X.; Zhang, W.; Chen, W.; Xu, X.; Wang, M.; Cheng, Y.-B. Hydrothermal synthesis of ultrasmall CuCrO2 nanocrystal alternatives to NiO nanoparticles in efficient p-type dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 24760–24768. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, H.; Chueh, C.-C.; Chen, W.; Yang, S.; Jen, A.K.-Y. Low-Temperature Solution-Processed CuCrO2 Hole-Transporting Layer for Efficient and Photostable Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702762. [Google Scholar] [CrossRef]
- Dunlap-Shohl, W.A.; Daunis, T.B.; Wang, X.; Wang, J.; Zhang, B.; Barrera, D.; Yan, Y.; Hsu, J.W.P.; Mitzi, D.B. Room-temperature fabrication of a delafossite CuCrO2 hole transport layer for perovskite solar cells. J. Mater. Chem. A 2018, 6, 469–477. [Google Scholar] [CrossRef]
- Yang, B.; Ouyang, D.; Huang, Z.; Ren, X.; Zhang, H.; Choy, W.C.H. Multifunctional Synthesis Approach of In:CuCrO2 Nanoparticles for Hole Transport Layer in High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1902600. [Google Scholar] [CrossRef]
- Akin, S.; Liu, Y.; Dar, M.I.; Zakeeruddin, S.M.; Grätzel, M.; Turan, S.; Sonmezoglu, S. Hydrothermally processed CuCrO2 nanoparticles as an inorganic hole transporting material for low-cost perovskite solar cells with superior stability. J. Mater. Chem. A 2018, 6, 20327–20337. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Jiang, G.S.; Su, J.R.; Zhu, C.F. The structural and electrical property of CuCr1−xNixO2 delafossite compounds. Mater. Lett. 2006, 60, 3871–3873. [Google Scholar] [CrossRef]
- Nagarajan, R.; Draeseke, A.D.; Sleight, A.W.; Tate, J. P-type conductivity in CuCr1−xMgxO2 films and powders. J. Appl. Phys. 2001, 89, 8022–8025. [Google Scholar] [CrossRef]
- Asemi, M.; Ghanaatshoar, M. Conductivity improvement of CuCrO2 nanoparticles by Zn doping and their application in solid-state dye-sensitized solar cells. Ceram. Int. 2016, 42, 6664–6672. [Google Scholar] [CrossRef]
- Schiavo, E.; Latouche, C.; Barone, V.; Crescenzi, O.; Pavone, M. An ab initio study of Cu-based delafossites as an alternative to nickel oxide in photocathodes: Effects of Mg-doping and surface electronic features. Phys. Chem. Chem. Phys. 2018, 20, 14082. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.-J.; Zhu, J.-Z.; Zhou, J.; Mo, M. Defect properties of CuCrO2: A density functional theory calculation. Chin. Phys. B 2012, 21, 087105. [Google Scholar] [CrossRef]
- Bywalez, R.; Götzendörfer, S.; Löbmann, P. Structural and physical effects of Mg-doping on p-type CuCrO2 and CuAl0.5Cr0.5O2 thin films. J. Mater. Chem. 2010, 20, 6562–6570. [Google Scholar] [CrossRef]
- Kaya, İ.C.; Sevindik, M.A.; Akyıldız, H. Characteristics of Fe- and Mg-doped CuCrO2 nanocrystals prepared by hydrothermal synthesis. J. Mater. Sci. Mater. Electron. 2016, 27, 2404–2411. [Google Scholar] [CrossRef]
- Monteiro, J.F.H.L.; Monteiro, F.C.; Jurelo, A.R.; Mosca, D.H. Conductivity in (Ag, Mg)-doped delafossite oxide CuCrO2. Ceram. Int. 2018, 44, 14101–14107. [Google Scholar] [CrossRef]
- Asemi, M.; Ghanaatshoar, M. Influence of TiO2 particle size and conductivity of the CuCrO2 nanoparticles on the performance of solid-state dye-sensitized solar cells. Bull. Mater. Sci. 2017, 40, 1379–1388. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, W.; Zeng, X.; Xu, Z.; Chen, W.; Cui, J.; Wang, M.; Sun, L.; Cheng, Y.-B. Enhanced Performance of p-Type Dye-Sensitized Solar Cells Based on Ultrasmall Mg-Doped CuCrO2 Nanocrystals. ChemSusChem 2013, 6, 1432–1437. [Google Scholar] [CrossRef]
- Jeong, S.; Seo, S.; Shin, H. p-Type CuCrO2 particulate films as the hole transporting layer for CH3NH3PbI3 perovskite solar cells. RSC Adv. 2018, 8, 27956–27962. [Google Scholar] [CrossRef]
- Cao, F.-Y.; Tseng, C.-C.; Lin, F.-Y.; Chen, Y.; Yan, H.; Cheng, Y.-J. Selenophene-Incorporated Quaterchalcogenophene-Based Donor–Acceptor Copolymers to Achieve Efficient Solar Cells with Jsc Exceeding 20 mA/cm2. Chem. Mater. 2017, 29, 10045–10052. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Seitz, O.; Peng, W.; Gartstein, Y.N.; Chabal, Y.J.; Malko, A.V. Efficient Radiative and Nonradiative Energy Transfer from Proximal CdSe/ZnS Nanocrystals into Silicon Nanomembranes. ACS Nano 2012, 6, 5574–5582. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Jansen, E.; Schäfer, W.; Will, G. R values in analysis of powder diffraction data using Rietveld refinement. J. Appl. Cryst. 1994, 27, 492–496. [Google Scholar] [CrossRef]
- Boekestein, A.; Stadhouders, A.M.; Stols, A.L.H.; Roomans, G.M. A comparison of ZAF-correction methods in quantitative X-ray microanalysis of light-element specimens. Ultramicroscopy 1983, 12, 65–68. [Google Scholar] [CrossRef]
- Miclau, M.; Ursu, D.; Kumar, S.; Grozescu, I. Hexagonal polytype of CuCrO2 nanocrystals obtained by hydrothermal method. J. Nanopart. Res. 2012, 14, 1110. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for chromium at 25–300 °C. Corros. Sci. 1997, 39, 43–57. [Google Scholar] [CrossRef]
- Yu, S.-H.; Yoshimura, M. Direct fabrication of ferrite MFe2O4 (M = Zn, Mg)/Fe composite thin films by soft solution processing. Chem. Mater. 2000, 12, 3805–3810. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for copper at 25 to 300°C. J. Electrochem. Soc. 1997, 144, 3476–3483. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Buford, M.C.; Hamilton, R.F.; Holian, A. A comparison of dispersing media for various engineered carbon nanoparticles. Part. Fibre Toxicol. 2007, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Thampy, S.; Ibarra, V.; Lee, Y.-J.; McCool, G.; Cho, K.; Hsu, J.W.P. Effects of synthesis conditions on structure and surface properties of SmMn2O5 mullite-type oxide. Appl. Surf. Sci. 2016, 385, 490–497. [Google Scholar] [CrossRef]
- Cano, E.; Torres, C.L.; Bastidas, J.M. An XPS study of copper corrosion originated by formic acid vapour at 40% and 80% relative humidity. Mater. Corros. 2001, 52, 667–676. [Google Scholar] [CrossRef]
- Ursu, D.; Miclau, M. Thermal stability of nanocrystalline 3R-CuCrO2. J. Nanopart. Res. 2014, 16, 2160. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Meyer, J.; Steirer, K.X.; Garcia, A.; Berry, J.J.; Ginley, D.S.; Olson, D.C.; Kahn, A.; Armstrong, N.R. Evidence for near-Surface NiOOH Species in Solution-Processed NiOx Selective Interlayer Materials: Impact on Energetics and the Performance of Polymer Bulk Heterojunction Photovoltaics. Chem. Mater. 2011, 23, 4988–5000. [Google Scholar] [CrossRef]
- Dunlap-Shohl, W.A.; Younts, R.; Gautam, B.; Gundogdu, K.; Mitzi, D.B. Effects of Cd Diffusion and Doping in High-Performance Perovskite Solar Cells Using CdS as Electron Transport Layer. J. Phys. Chem. C 2016, 120, 16437–16445. [Google Scholar] [CrossRef]
- Qin, P.; He, Q.; Yang, G.; Yu, X.; Xiong, L.; Fang, G. Metal ions diffusion at heterojunction chromium Oxide/CH3NH3PbI3 interface on the stability of perovskite solar cells. Surf. Interfaces 2018, 10, 93–99. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Unraveling the Impacts Induced by Organic and Inorganic Hole Transport Layers in Inverted Halide Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 7055–7065. [Google Scholar] [CrossRef]
- Hoste, S.; Van De Vondel, D.F.; Van Der Kelen, G.P. XPS Spectra of organometallic phenyl compounds of P, As, Sb and Bi. J. Electron Spectrosc. Relat. Phenom. 1979, 17, 191–195. [Google Scholar] [CrossRef]
- Xu, F.; Ma, X.; Kauzlarich, S.M.; Navrotsky, A. Enthalpies of formation of CdSxSe1−x solid solutions. J. Mater. Res. 2009, 24, 1368–1374. [Google Scholar] [CrossRef]
- Materials Project. Available online: https://materialsproject.org/#apps/reactioncalculator/{%22reactants%22%3A[%22Cu%22%2C%22I%22]%2C%22products%22%3A[%22CuI%22]} (accessed on 7 September 2019).
- Materials Project. Available online: https://materialsproject.org/#apps/reactioncalculator/{%22reactants%22%3A[%22Cr%22%2C%22O%22]%2C%22products%22%3A[%22Cr2O3%22]} (accessed on 7 September 2019).
- Hoogewijs, R.; Fiermans, L.; Vennik, J. Electronic relaxation processes in the KLL′ auger spectra of the free magnesium atom, solid magnesium and MgO. J. Electron Spectrosc. Relat. Phenom. 1977, 11, 171–183. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Ryan, J.W.; Miyano, K. Exploring the effects of interfacial carrier transport layers on device performance and optoelectronic properties of planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 8819–8827. [Google Scholar] [CrossRef]
- Wang, J.; Fu, W.; Jariwala, S.; Sinha, I.; Jen, A.K.-Y.; Ginger, D.S. Reducing Surface Recombination Velocities at the Electrical Contacts Will Improve Perovskite Photovoltaics. ACS Energy Lett. 2019, 4, 222–227. [Google Scholar] [CrossRef]










| Sample | CCO | 5% Mg:CCO | 10% Mg:CCO |
|---|---|---|---|
| Polytype composition (%) | 3R-CCO = 59.1 ± 3.0 | 3R-CCO = 59.5 ± 2.6 | 3R-CCO = 56.0 ± 2.7 |
| 2H-CCO = 40.9 ± 3.0 | 2H-CCO = 40.5 ± 2.6 | 2H-CCO = 44.0 ± 2.7 | |
| Rwp (%) 1 | 13.3 | 13.7 | 13.4 |
| Rexp (%) 2 | 9.0 | 9.0 | 9.1 |
| Rp (%) 3 | 9.0 | 10.9 | 10.5 |
| Χ2 | 2.2 | 2.3 | 2.2 |
| Crystal size (nm) based on (004) | 7.8 ± 0.4 (2H-CCO) | 5.6 ± 0.3 (2H-CCO) | 4.5 ± 0.3 (2H-CCO) |
| Crystal size (nm) based on (110) | 9.6 ± 0.9 (3R-CCO) | 9.4 ± 0.9 (3R-CCO) | 8.7 ± 1.0 (3R-CCO) |
| 10.2 ± 1.0 (2H-CCO) | 12.3 ± 1.3 (2H-CCO) | 13.1 ± 1.5 (2H-CCO) | |
| Lattice parameter a 4 and c 5 (Å) for 3R-CCO | a = 2.99 | a = 2.99 | a = 3.00 |
| c =17.44 | c =17.44 | c =17.44 | |
| Lattice parameter a and c (Å) for 2H-CCO | a = 2.99 | a = 2.99 | a = 3.00 |
| c =11.43 | c =11.44 | c =11.46 |
| Sample | CCO | 5% Mg:CCO | 10% Mg:CCO |
|---|---|---|---|
| Mg/(Mg+Cr) (%) 1 | 0 | 4.0 ± 0.2 | 9.8 ± 1.3 |
| Nanoparticle size (nm) 2 | 10.3 ± 2.1 | 8.2 ± 2.1 | 9.8 ± 3.0 |
| Sample | Thickness (nm) | Direct Eg (eV) 1 | WFmedian – IEmedian (eV) |
|---|---|---|---|
| CCO | 18 | 3.27 ± 0.02 | 0.08 |
| 5% Mg:CCO | 18 | 3.25 ± 0.03 | 0.09 |
| 10% Mg:CCO | 18 | 3.27 ± 0.03 | 0.16 |
| Device Type 1 | HTL_Type | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|---|
| P3HT:PC61BM | CCO | 6.94± 0.15 | 0.570 ± 0.000 | 0.685 ± 0.008 | 2.71 ± 0.06 |
| 5% Mg:CCO | 7.04 ± 0.11 | 0.583 ± 0.005 | 0.642 ± 0.022 | 2.63 ± 0.08 | |
| 10% Mg:CCO | 7.06 ± 0.11 | 0.581 ± 0.007 | 0.666 ± 0.017 | 2.73 ± 0.03 | |
| PFBT2Se2Th:PC71BM | CCO | 10.50 ± 0.29 | 0.666 ± 0.007 | 0.684 ± 0.014 | 4.78 ± 0.18 |
| 5% Mg:CCO | 10.77 ± 0.61 | 0.664 ± 0.007 | 0.689 ± 0.011 | 4.93 ± 0.26 | |
| 10% Mg:CCO | 10.88 ± 0.50 | 0.665 ± 0.007 | 0.678 ± 0.011 | 4.91 ± 0.27 | |
| PTB7-Th:ITIC | CCO | 11.55 ± 0.17 | 0.786 ± 0.007 | 0.548 ± 0.010 | 4.97 ± 0.14 |
| 5% Mg:CCO | 11.87 ± 0.15 | 0.793 ± 0.005 | 0.559 ± 0.003 | 5.26 ± 0.08 | |
| 10% Mg:CCO | 12.02 ± 0.27 | 0.785 ± 0.007 | 0.541 ± 0.011 | 5.11 ± 0.22 | |
| MAPbI3 PSC (forward scan) | CCO | 18.91± 0.43 | 0.985 ± 0.058 | 0.678 ± 0.025 | 12.64 ± 0.99 |
| 5% Mg:CCO | 19.26 ± 0.54 | 1.003 ± 0.010 | 0.696 ± 0.023 | 13.45 ± 0.44 | |
| 10% Mg:CCO | 19.40 ± 0.39 | 1.007 ± 0.014 | 0.703 ± 0.018 | 13.73 ± 0.34 | |
| MAPbI3 PSC (reverse scan) | CCO | 18.70± 0.31 | 1.012 ± 0.006 | 0.697 ± 0.031 | 13.19 ± 0.71 |
| 5% Mg:CCO | 19.20 ± 0.41 | 1.011 ± 0.004 | 0.719 ± 0.012 | 13.96 ± 0.33 | |
| 10% Mg:CCO | 19.37 ± 0.35 | 1.014 ± 0.006 | 0.719 ± 0.012 | 14.12 ± 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Thampy, S.; Dunlap-Shohl, W.A.; Xu, W.; Zheng, Y.; Cao, F.-Y.; Cheng, Y.-J.; Malko, A.V.; Mitzi, D.B.; Hsu, J.W.P. Mg Doped CuCrO2 as Efficient Hole Transport Layers for Organic and Perovskite Solar Cells. Nanomaterials 2019, 9, 1311. https://doi.org/10.3390/nano9091311
Zhang B, Thampy S, Dunlap-Shohl WA, Xu W, Zheng Y, Cao F-Y, Cheng Y-J, Malko AV, Mitzi DB, Hsu JWP. Mg Doped CuCrO2 as Efficient Hole Transport Layers for Organic and Perovskite Solar Cells. Nanomaterials. 2019; 9(9):1311. https://doi.org/10.3390/nano9091311
Chicago/Turabian StyleZhang, Boya, Sampreetha Thampy, Wiley A. Dunlap-Shohl, Weijie Xu, Yangzi Zheng, Fong-Yi Cao, Yen-Ju Cheng, Anton V. Malko, David B. Mitzi, and Julia W. P. Hsu. 2019. "Mg Doped CuCrO2 as Efficient Hole Transport Layers for Organic and Perovskite Solar Cells" Nanomaterials 9, no. 9: 1311. https://doi.org/10.3390/nano9091311
APA StyleZhang, B., Thampy, S., Dunlap-Shohl, W. A., Xu, W., Zheng, Y., Cao, F.-Y., Cheng, Y.-J., Malko, A. V., Mitzi, D. B., & Hsu, J. W. P. (2019). Mg Doped CuCrO2 as Efficient Hole Transport Layers for Organic and Perovskite Solar Cells. Nanomaterials, 9(9), 1311. https://doi.org/10.3390/nano9091311





