Preparation, Characterization, and Performance Analysis of S-Doped Bi2MoO6 Nanosheets
Abstract
:1. Introduction
2. Experiments
2.1. Preparation of Photocatalyst
2.2. Characterization
2.3. Determination of Photocatalytic Property
2.4. Determination of Photoelectrochemical Property
3. Results and Discussion
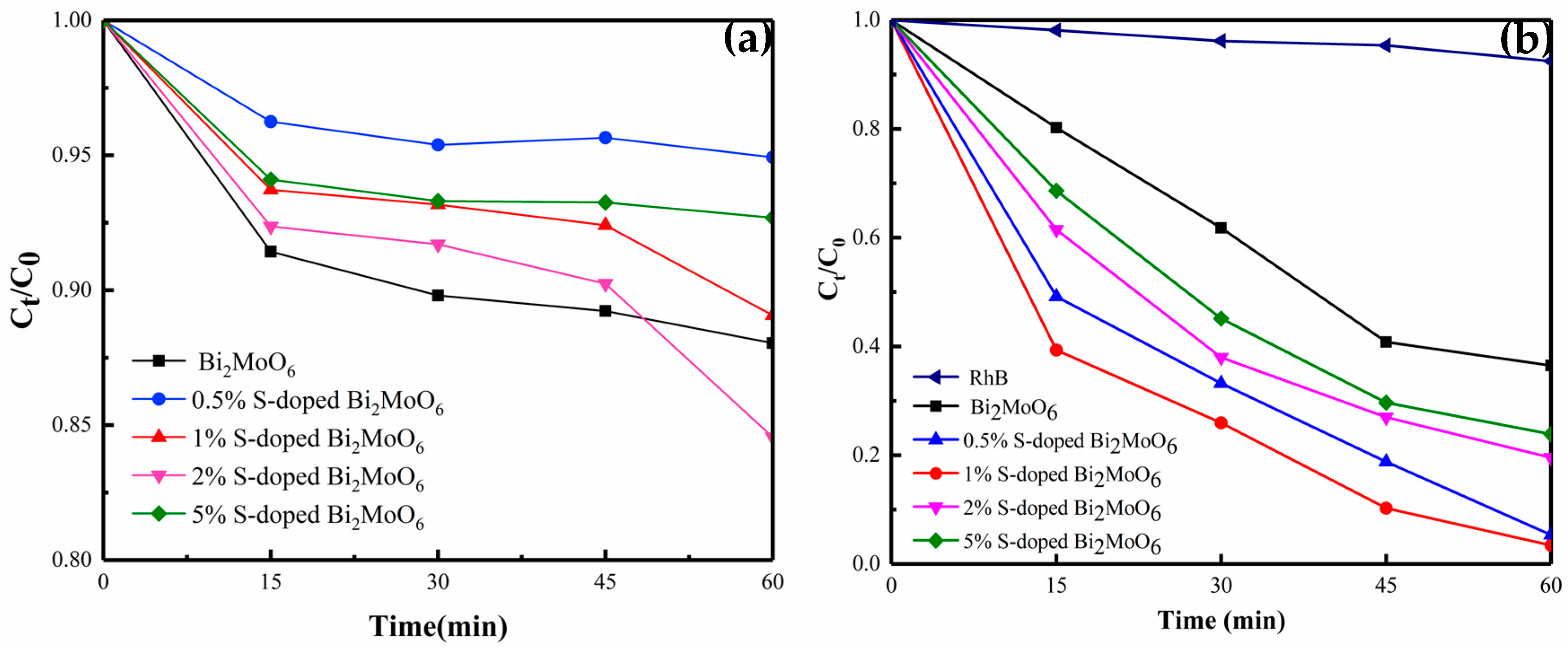
3.1. Photocatalytic Property
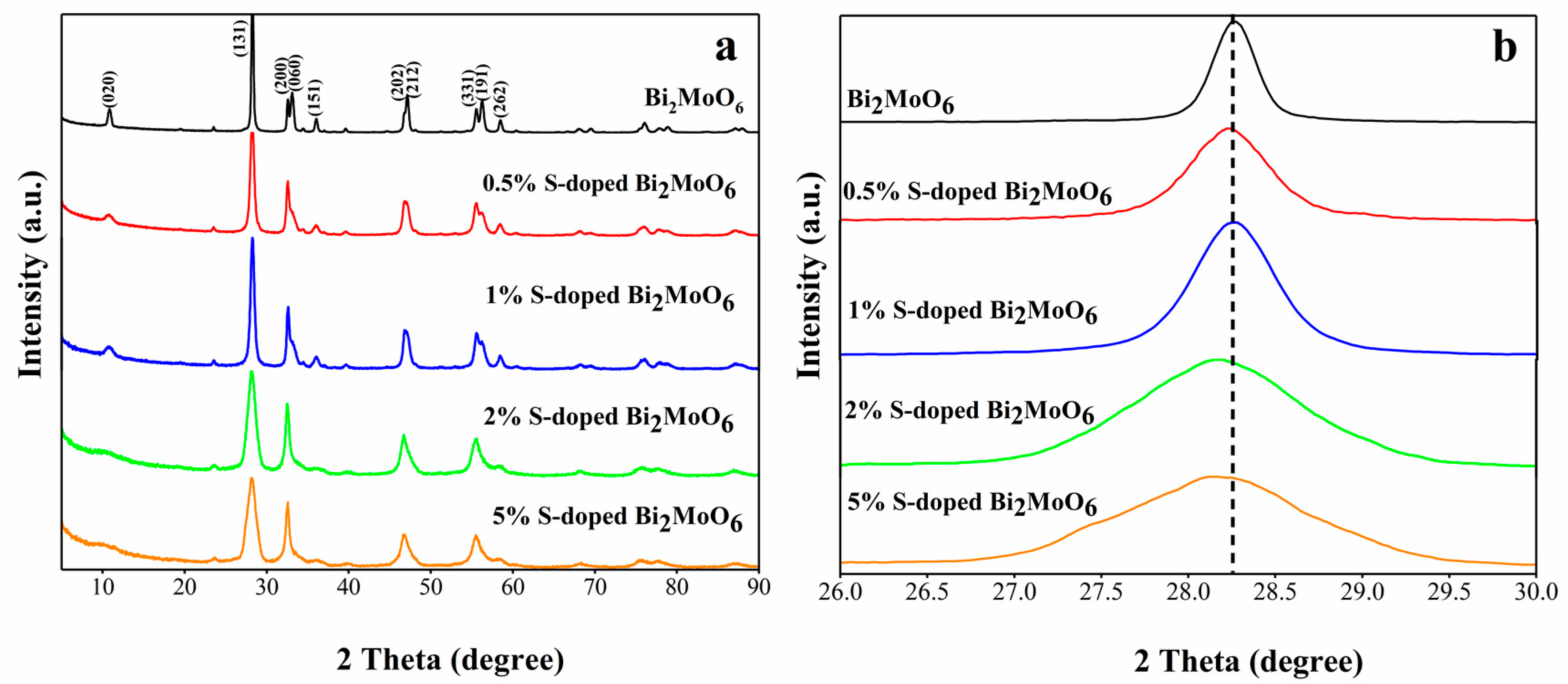
3.2. Crystal Structure and Composition
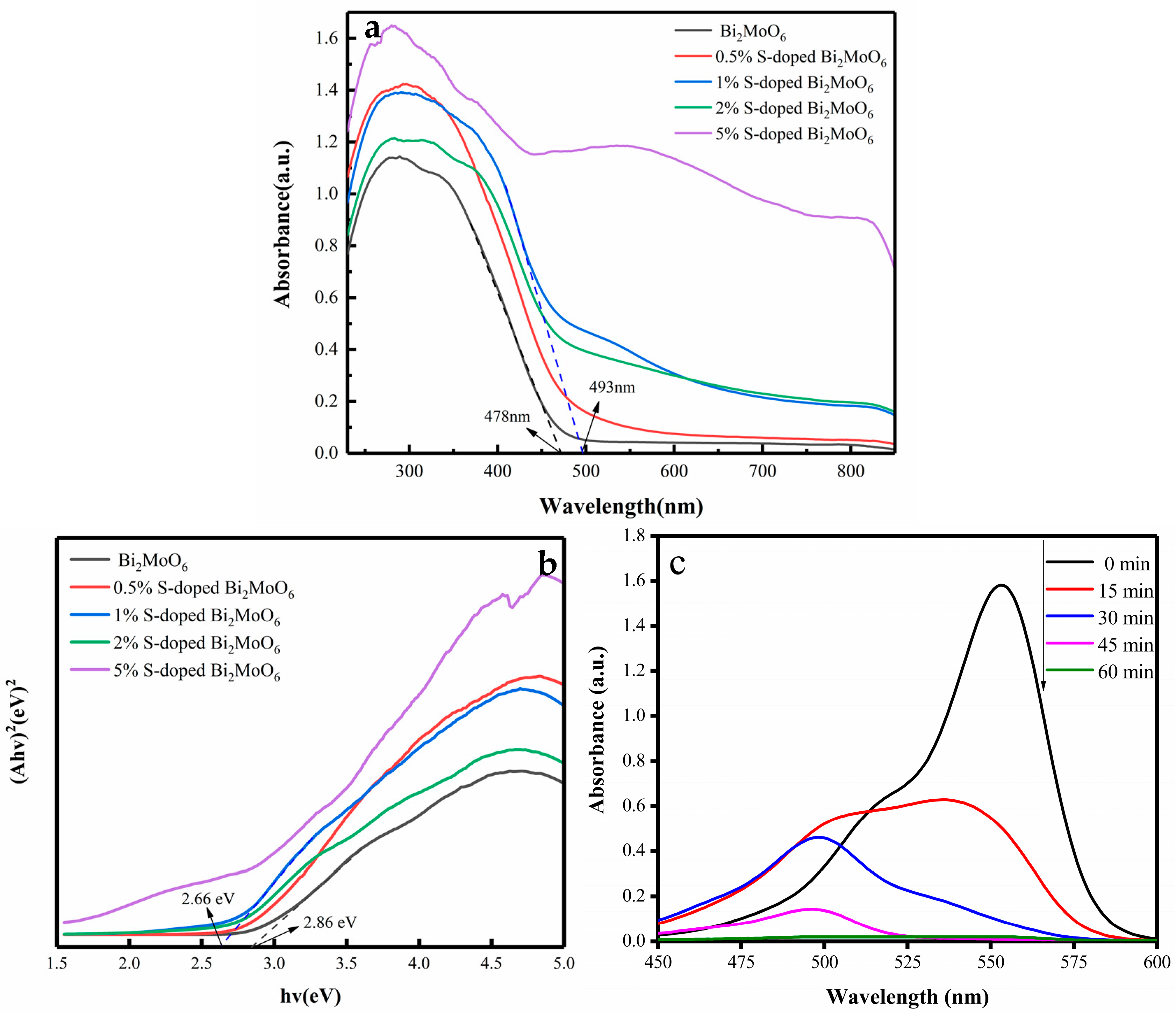
3.3. Optical Property
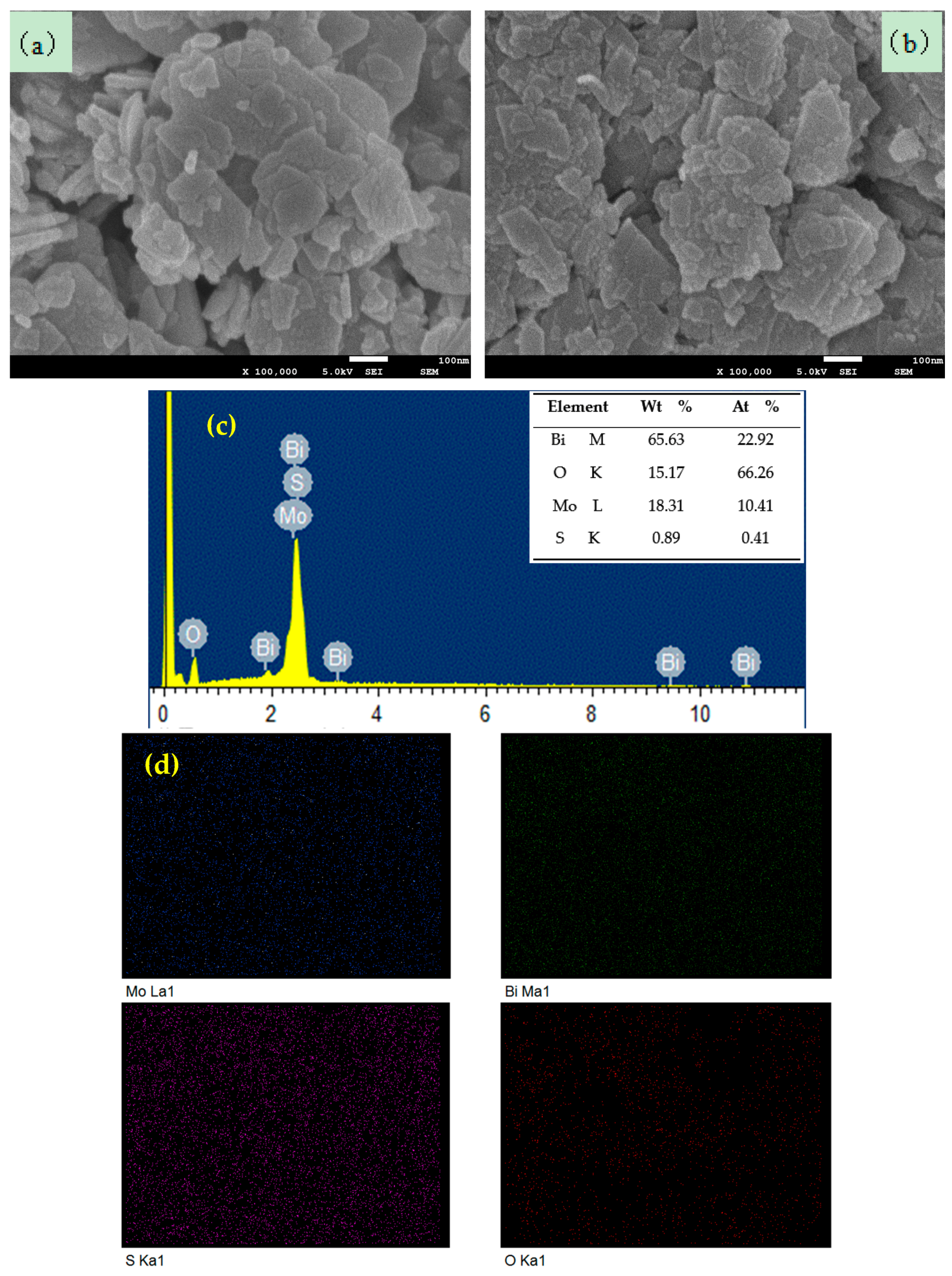
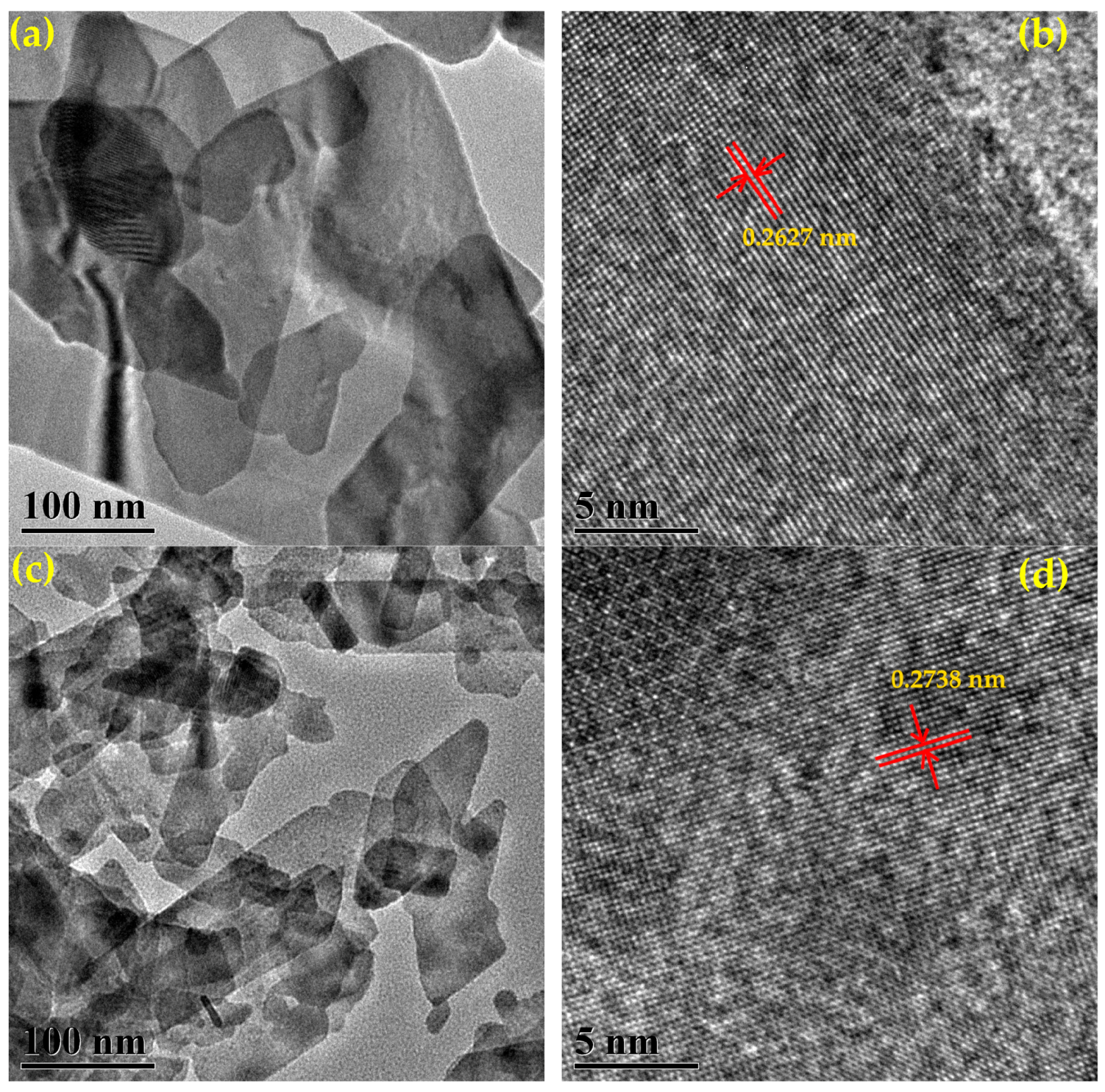
3.4. Morphology and Crystal Analysis
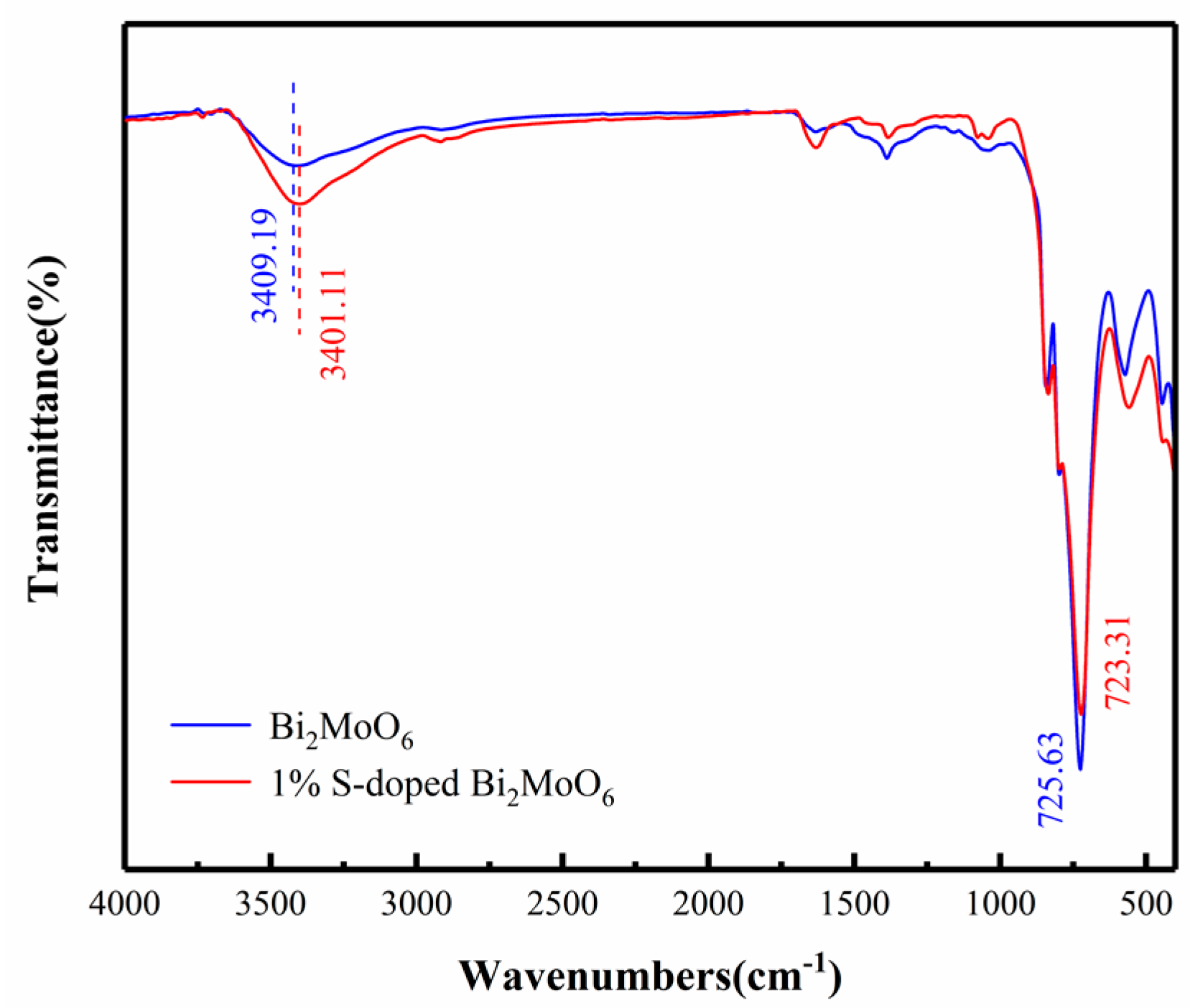
3.5. Chemical Composition and Valence Analysis
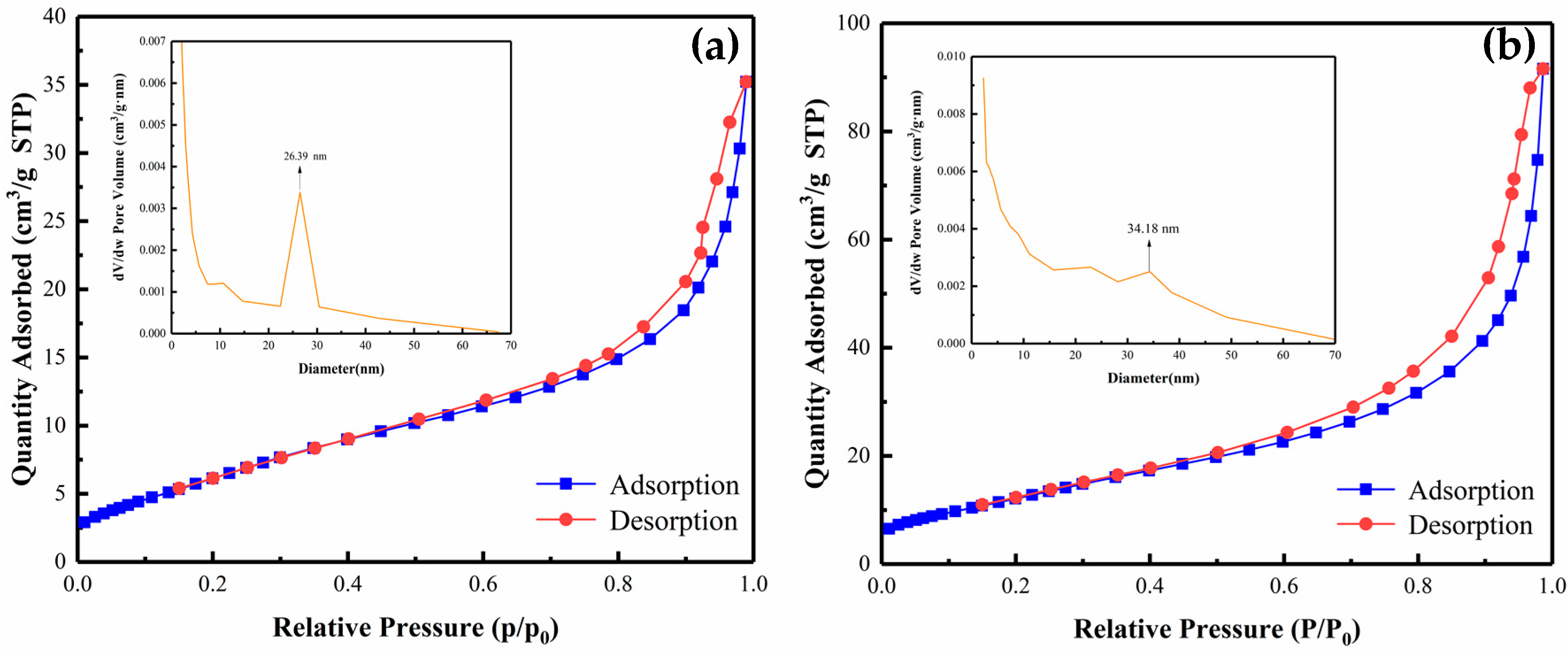
3.6. Brunauer Emmett Teller (BET) Specific Surface Area Analysis
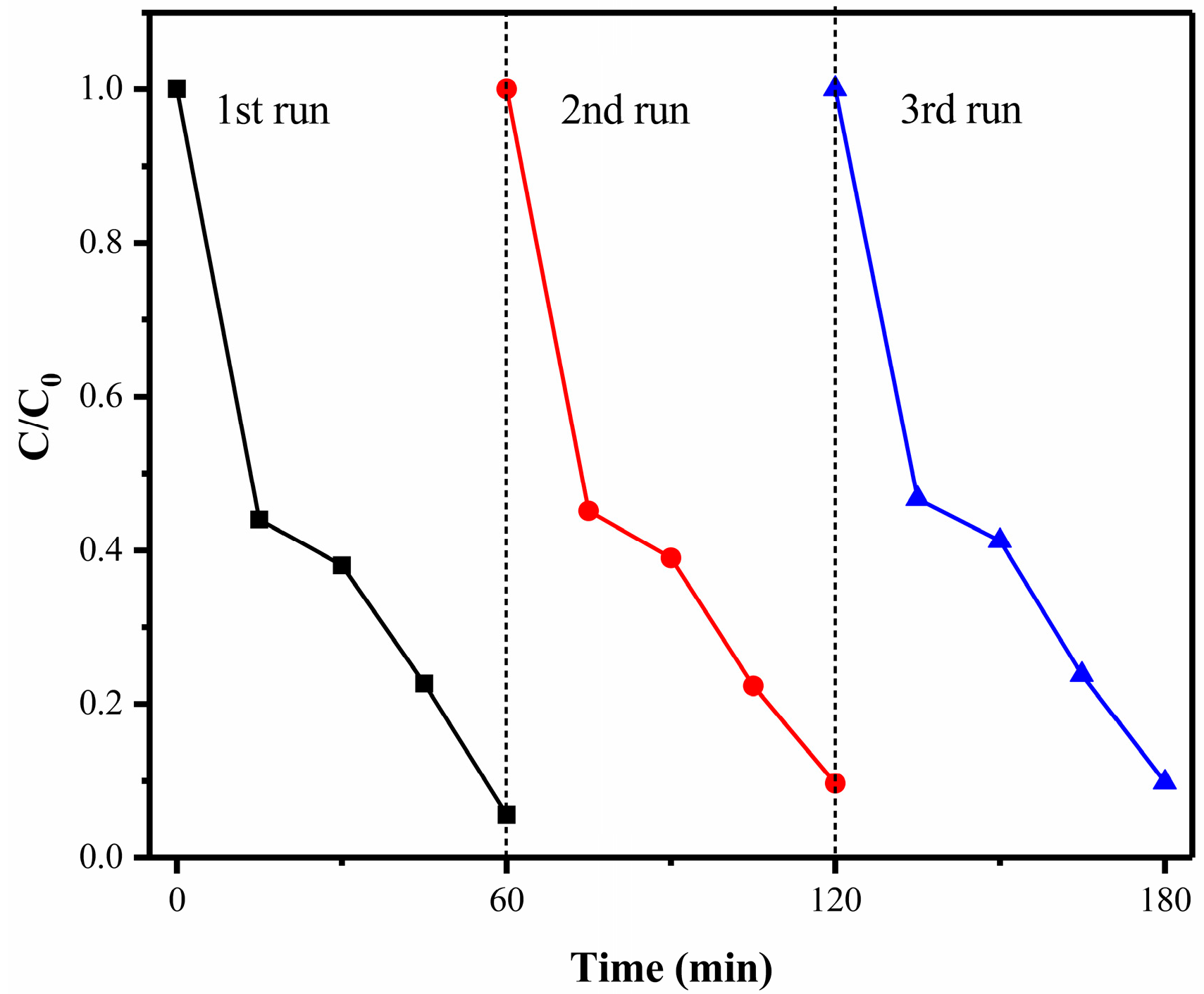
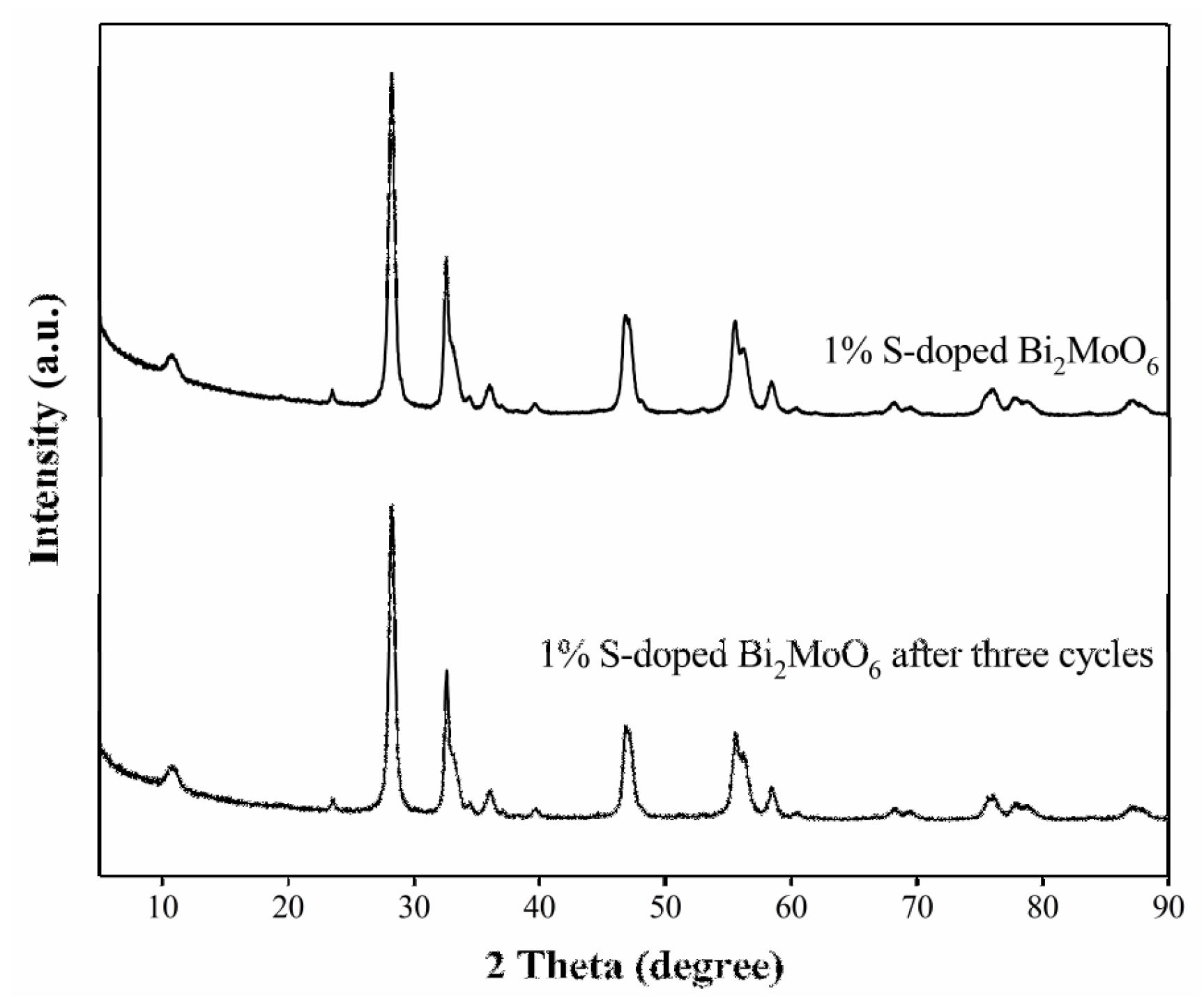
3.7. Stability and Reuse Property
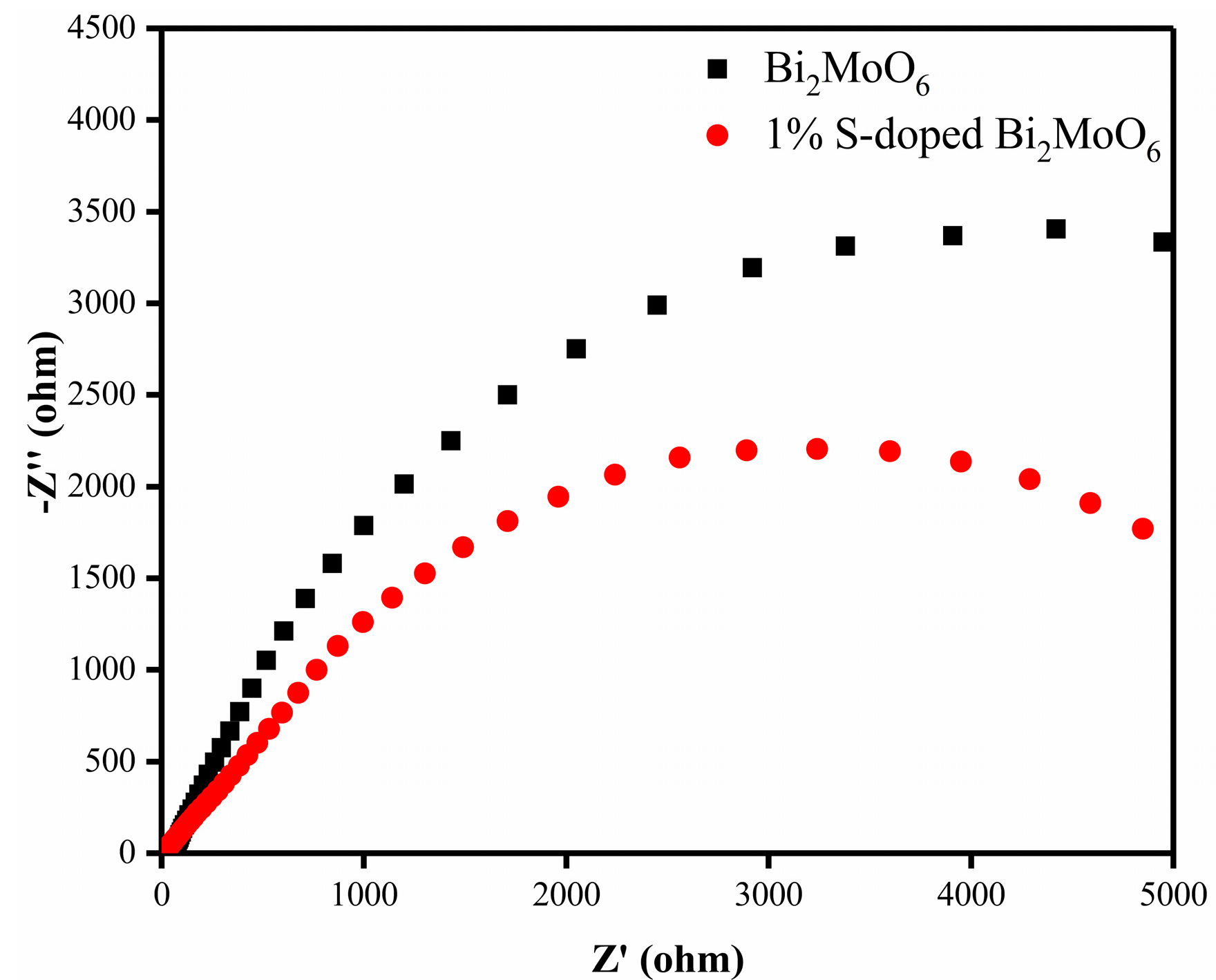
3.8. Photoelectrochemical Property
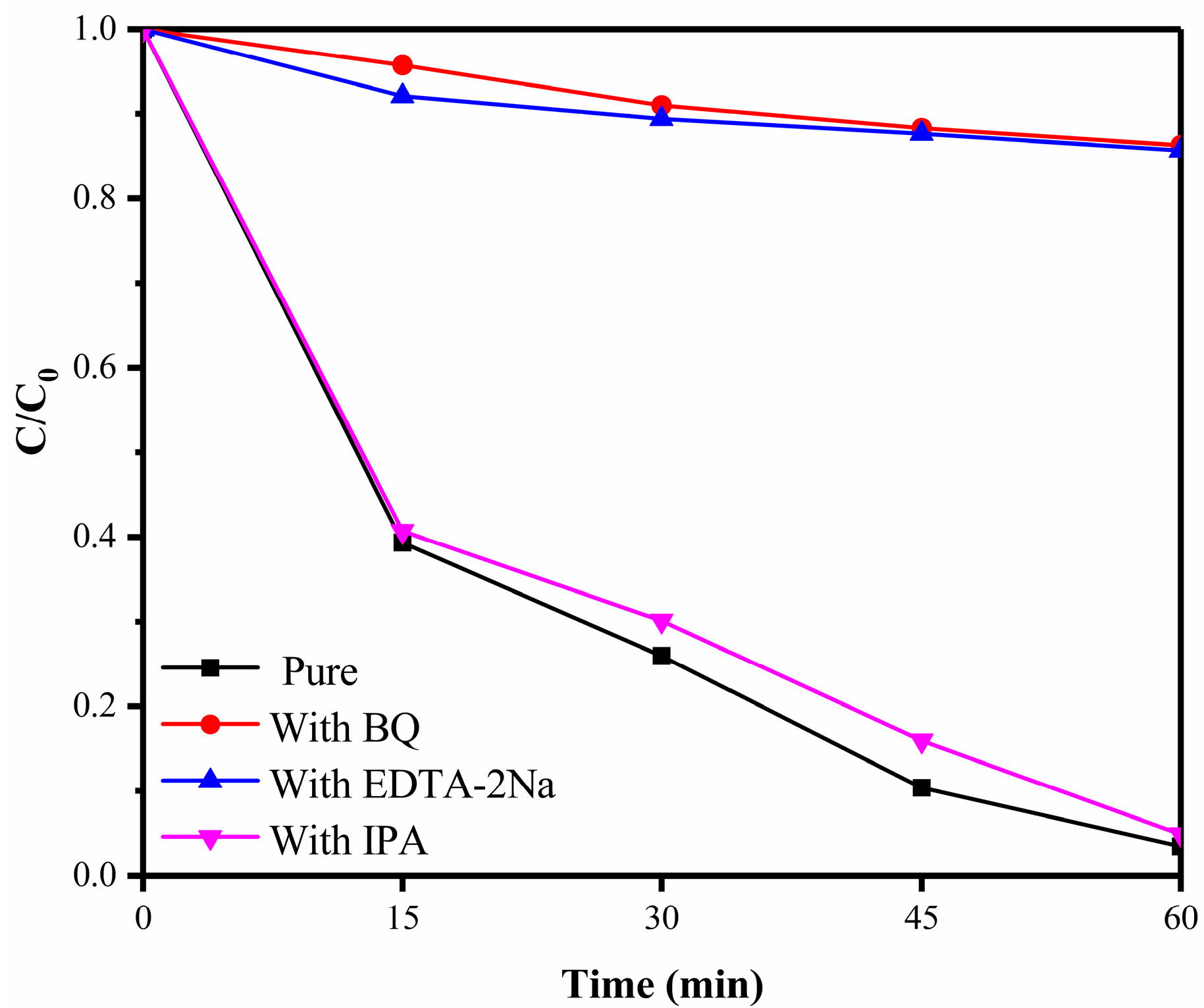
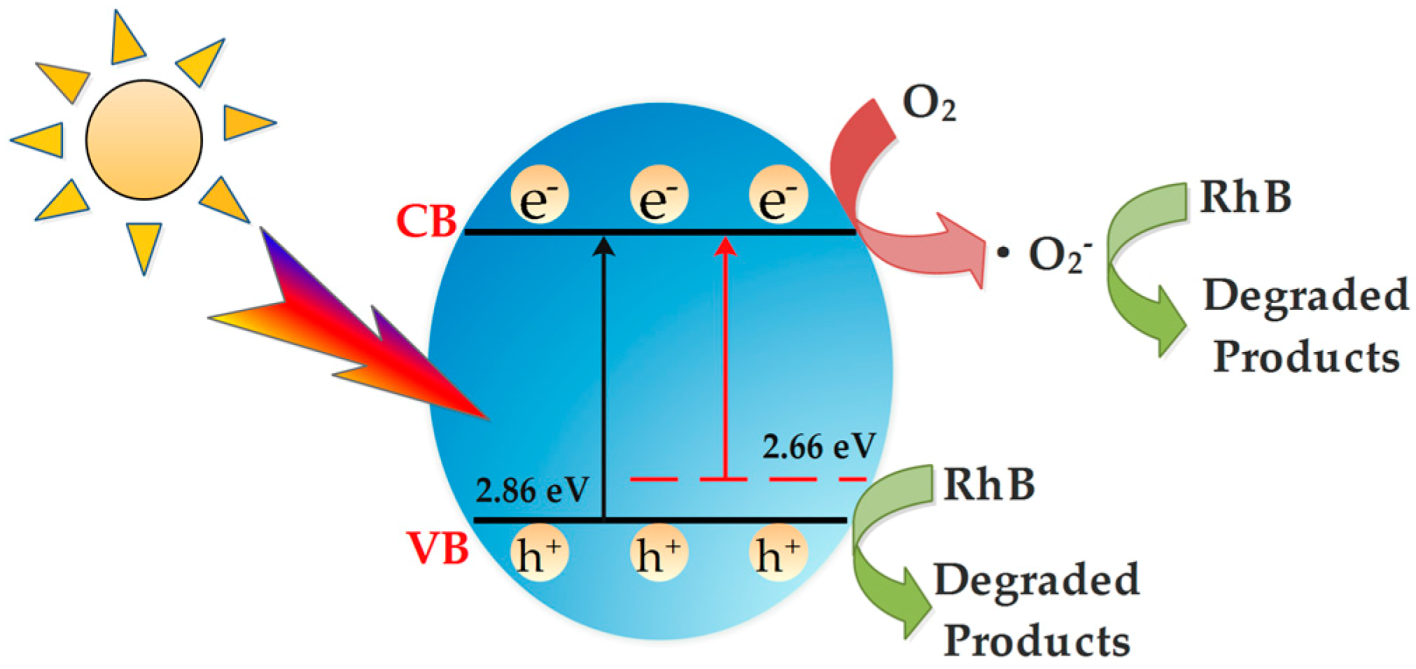
3.9. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photocatalysis of water at a semiconductor electrode. Nature 1972, 37, 238–245. [Google Scholar]
- Dong, S.Y.; Feng, J.L.; Fan, M.H.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Wang, H.L.; Xu, L.J.; Liu, C.L.; Lu, Y.; Feng, Q.; Wu, T.; Wang, R. Composite magnetic photocatalyst Bi5O7I/MnxZn1-xFe2O4: Hydrothermal-roasting preparation and enhanced photocatalytic activity. Nanomaterials 2019, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, W.; Shang, M.; Sun, S.; Gao, E. Heterostructured bismuth molybdate composite: Preparation and improved photocatalytic activity under visible-light irradiation. ACS Appl. Mater. Interfaces 2011, 3, 2529–2533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, J.F.; Zhou, S.; Ouyang, H.B.; Cao, L.Y.; Li, A.T. Microwave hydrothermal synthesis and optical properties of flower-like Bi2MoO6 crystallites. Ceram. Int. 2013, 39, 7391–7394. [Google Scholar] [CrossRef]
- Li, H.H.; Li, K.W.; Wang, H. Hydrothermal synthesis and photocatalytic properties of bismuth molybdate materials. Mater. Chem. Phys. 2009, 116, 134–142. [Google Scholar] [CrossRef]
- Meng, X.C.; Zhang, Z.S. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Jia, Y.L.; Ma, Y.; Tang, J.Z.; Shi, W. Hierarchical nanosheet-based Bi2MoO6 microboxes for efficient photocatalytic performance. Dalton Trans. 2018, 47, 5542–5547. [Google Scholar] [CrossRef]
- Zhou, T.F.; Hu, J.C.; Li, J.L. Er3+ doped bismuth molybdate nanosheets with exposed {010} facets and enhanced photocatalytic performance. Appl. Catal. B Environ. 2011, 110, 221–230. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Lu, Q.F.; Wei, M.Z.; Wei, M.; Guo, E.; Yao, L.; Sun, K. ZnO/γ-Bi2MoO6 heterostructured nanotubes: Electrospinning fabrication and highly enhanced photoelectrocatalytic properties under visible-light irradiation. J. Sol-Gel. Sci. Technol. 2018, 85, 84–92. [Google Scholar] [CrossRef]
- Zhong, Y.; He, Z.T.; Chen, D.M.; Hao, D.; Hao, W. Enhancement of photocatalytic activity of Bi2MoO6 by fluorine substitution. Appl. Surf. Sci. 2019, 467, 740–748. [Google Scholar] [CrossRef]
- Wang, P.F.; Ao, Y.H.; Wang, C.; Hou, J.; Qian, J. A one-pot method for the preparation of graphene—Bi2MoO6 hybrid photocatalysts that are responsive to visible-light and have excellent photocatalytic activity in the degradation of organic pollutants. Carbon 2012, 50, 5256–5264. [Google Scholar] [CrossRef]
- Xing, Y.X.; Zhang, J.; Liu, Z.L.; Du, C.F. Steering photoinduced charge kinetics via anionic group doping in Bi2MoO6 for efficient photocatalytic removal of water organic pollutants. RSC Adv. 2017, 7, 35883–35896. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Guo, P.Y.; Sun, M.Z.; Zhang, Y.; Tong, Z.; You, M.; Lv, C. Hydrothermal synthesis of B-doped Bi2MoO6 and its high photocatalytic performance for the degradation of Rhodamine B. J. Phys. Chem. Solids 2018, 113, 86–93. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Guo, J.; Zhou, L.X.; Lan, Y.Q. Sulfur-doped copper-cobalt bimetallic oxides with abundant Cu (I): A novel peroxymonosulfate activator for chloramphenicol degradation. Chem. Eng. J. 2019, 361, 1304–1316. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.G.; Wu, C.C.; Cui, Z.; Rao, P.H. Enhancing photocatalytic activity of Bi2MoO6 via surface co-doping with Ni2+ and Ti4+ ions. J. Phys. Chem. Solids 2019, 129, 209–216. [Google Scholar] [CrossRef]
- Ding, X.; Ho, W.K.; Shang, J.; Zhang, L.Z. Self doping promoted photocatalytic removal of no under visible light with Bi2MoO6: Indispensable role of superoxide ions. Appl. Catal. B Environ. 2016, 182, 316–325. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, H.R.; Jiang, H.T.; Yu, F.; Shang, Z.R. Hydrothermal Synthesis and Characterization of Ce3+ Doped Bi2MoO6 for Water Treatment. Catal. Lett. 2019, 1–11. [Google Scholar] [CrossRef]
- Dai, Z.; Qin, F.; Zhao, H.P.; Ding, J.; Liu, Y.L.; Chen, R. Crystal defect engineering of aurivillius Bi2MoO6 by Ce doping for increased reactive species production in photocatalysis. ACS Catal. 2016, 6, 3180–3192. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.Y.; Li, W.B.; Zhang, F.; Li, X.B.; Xu, L.K.; Sun, M.X. First-principle calculation of the electronic structures and optical properties of the metallic and nonmetallic elements-doped ZnO on the basis of photocatalysis. Physica B 2019, 555, 53–60. [Google Scholar] [CrossRef]
- Xing, Y.X.; Gao, X.C.; Ji, G.F.; Liu, Z.L.; Du, C.F. Synthesis of carbon doped Bi2MoO6 for enhanced photocatalytic performance and tumor photodynamic therapy efficiency. Appl. Surf. Sci. 2019, 465, 369–382. [Google Scholar] [CrossRef]
- Jin, S.S.; Hao, H.S.; Gan, Y.J.; Guo, W.; Li, H.; Hu, X.; Hou, H.; Zhang, G.; Yan, S.; Gao, W.; et al. Preparation and improved photocatalytic activities of Ho3+/Yb3+ co-doped Bi2MoO6. Mater. Chem. Phys. 2017, 199, 107–112. [Google Scholar] [CrossRef]
- Dai, W.L.; Hu, X.; Wang, T.Y.; Xiong, W.W.; Luo, X.B.; Zou, J.P. Hierarchical CeO2/Bi2MoO6 heterostructured nanocomposites for photoreduction of CO2 into hydrocarbons under visible light irradiation. Appl. Surf. Sci. 2018, 434, 481–491. [Google Scholar] [CrossRef]
- Li, H.D.; Li, W.J.; Gu, S.N.; Wang, F.Z.; Liu, X.T.; Ren, C.J. Forming oxygen vacancies inside in lutetium-doped Bi2MoO6 nanosheets for enhanced visible-light photocatalytic activity. Mol. Catal. 2017, 433, 301–312. [Google Scholar] [CrossRef]
- Dutta, D.P.; Ballal, A.; Chopade, S.; Kumar, A. A study on the effect of transition metal (Ti4+, Mn2+, Cu2+ and Zn2+)-doping on visible light photocatalytic activity of Bi2MoO6 nanorods. J. Photochem. Photobiol. A 2017, 346, 105–112. [Google Scholar] [CrossRef]
- Li, H.D.; Li, W.J.; Gu, S.N.; Wang, F.Z.; Zhou, H.L.; Liu, X.T.; Ren, C.J. Enhancement of photocatalytic activity in Tb/Eu co-doped Bi2MoO6: The synergistic effect of Tb–Eu redox cycles. RSC Adv. 2016, 6, 48089–48098. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Ekthammathat, N.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis, structure, and optical properties of pure and silver-doped Bi2MoO6 nanoplates. Russ. J. Phys. Chem. A 2015, 89, 2443–2448. [Google Scholar] [CrossRef]
- Umapathy, V.; Manikandan, A.; Antony, S.A.; Ramu, P.; Neeraja, P. Structure, morphology and opto-magnetic properties of Bi2MoO6 nano-photocatalyst synthesized by sol-gel method. Trans. Nonferrous Met. Soc. China 2015, 25, 3271–3278. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhang, L.; Hu, J.S.; Huang, X.H. Facile hydrothermal synthesis and improved photocatalytic activities of Zn2+ doped Bi2MoO6 nanosheets. RSC Adv. 2016, 6, 32349–32357. [Google Scholar] [CrossRef]
- Yu, C.L.; Wu, Z.; Liu, R.Y.; He, H.B.; Fan, W.H.; Xue, S.S. The effects of Gd3+ doping on the physical structure and photocatalytic performance of Bi2MoO6 nanoplate crystals. J. Phys. Chem. Solids 2016, 93, 7–13. [Google Scholar] [CrossRef]
- Meng, X.C.; Zhang, Z.S. Pd-doped Bi2MoO6 plasmonic photocatalysts with enhanced visible light photocatalytic performance. Appl. Surf. Sci. 2017, 392, 169–180. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, X.L.; Zhang, X.H.; Ding, X.; Yang, Z.X.; Dai, K.; Chen, H. A plate-on-plate sandwiched Z-scheme heterojunction photocatalyst: BiOBr-Bi2MoO6 with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 391, 194–201. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zheng, T.T.; Xu, J.Y.; Zeng, H.B.; Zhang, N. Carbon quantum dots/Bi2MoO6 composites with photocatalytic H2 evolution and near infrared activity. J. Photochem. Photobiol. A 2017, 346, 24–31. [Google Scholar] [CrossRef]
- Xu, C.Q.; Qian, H.Z.; Yong, H.B.; Huang, X.G.; Wang, W.; Wen, Y.; Zhong, K.; Zhou, Y.; Lai, M. Hydrothermal synthesis of BiVO4/Bi2MoO6 composites with enhanced photocatalytic activity. Int. J. Mod. Phys. B 2017, 31, 1744059. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, Z.D.; Ma, Z. Facile Formation of Bi2O2CO3/Bi2MoO6 Nanosheets for Visible Light-Driven Photocatalysis. ACS Omega 2019, 4, 3871–3880. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.X.; Cui, E.T.; Liu, H.X.; Wu, J.; Dai, Y.; Yu, G.Y. Layered Bi2MoO6/LDH hetero-structured composites with enhanced visible light photocatalytic activity. J. Mater. Sci. 2019, 30, 2572–2584. [Google Scholar] [CrossRef]
- Jia, Y.L.; Ma, Y.; Zhu, L.L.; Dong, J.; Lin, Y.H. Hierarchical NiCo2O4/Bi2MoO6 heterostructured nanorod arrays for high-performance supercapacitors. Mater. Lett. 2019, 244, 130–133. [Google Scholar] [CrossRef]
- Zhen, H.J.; Khan, M.A.; Xia, M.Z.; Lei, W.; Wang, F.Y. Controllable synthesis of flower-root shaped Bi2O3/Bi2MoO6 heterostructures as an efficient photocatalyst under visible light irradiation. J. Photochem. Photobiol. A 2019, 372, 78–88. [Google Scholar] [CrossRef]
- Zhang, G.P.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Zeng, D.B.; Yu, C.L.; Huang, W.Y.; Yang, K.; Liu, X.Q.; Liu, H. Binary-phase TiO2 modified Bi2MoO6 crystal for effective removal of antibiotics under visible light illumination. Mater. Res. Bull. 2019, 112, 336–345. [Google Scholar] [CrossRef]
- Jia, Y.L.; Ma, Y.; Lin, Y.H.; Tang, J.Z.; Shi, W.B. Fabrication of Bi2MoO6/ZnO Heterojunction Nanosheet Array with High Photoelectrochemical Property. J. Nanosci. Nanotechnol. 2019, 19, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.F.; Zhang, J.F.; Dai, K.; Zhu, G.P.; Liang, C.H. A Z-scheme Bi2MoO6/CdSe-diethylenetriamine heterojunction for enhancing photocatalytic hydrogen production activity under visible light. Dalton Trans. 2019, 48, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Shen, H.D.; Sun, X.; Xue, W.W.; Shoneye, A.; Ma, J.; Luo, L.; Wang, D.; Wang, J.; Tang, J. Synergistic effect of surface oxygen vacancies and interfacial charge transfer on Fe(III)/Bi2MoO6 for efficient photocatalysis. Appl. Catal. B Environ. 2019, 247, 150–162. [Google Scholar] [CrossRef]
- Gao, X.; Ji, X.D.; Nguyen, T.T.; Gong, X.C.; Chai, R.S.; Guo, M.H. A novel composite material with wood-based carbon quantum dots modified Bi2MoO6 hollow microspheres. Vacuum 2019, 164, 256–264. [Google Scholar] [CrossRef]
- Li, Z.Z.; Meng, X.C.; Zhang, Z.S. Fewer-layer BN nanosheets-deposited on Bi2MoO6 microspheres with enhanced visible light-driven photocatalytic activity. Appl. Surf. Sci. 2019, 483, 572–580. [Google Scholar] [CrossRef]




| Sulfur Doping Amount | 0 | 0.5% | 1% | 2% | 5% |
|---|---|---|---|---|---|
| Average grain size (nm) | 28.2 | 17.7 | 16.1 | 10.8 | 9.2 |
| Band gap energy (eV) | 2.86 | 2.75 | 2.66 | 2.60 | 2.49 |
| Specific surface area (m2/g) | 26 | 31 | 49 | 50 | 54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Li, D.; Wang, H.; Liu, C.; Xu, L. Preparation, Characterization, and Performance Analysis of S-Doped Bi2MoO6 Nanosheets. Nanomaterials 2019, 9, 1341. https://doi.org/10.3390/nano9091341
Wang R, Li D, Wang H, Liu C, Xu L. Preparation, Characterization, and Performance Analysis of S-Doped Bi2MoO6 Nanosheets. Nanomaterials. 2019; 9(9):1341. https://doi.org/10.3390/nano9091341
Chicago/Turabian StyleWang, Ruiqi, Duanyang Li, Hailong Wang, Chenglun Liu, and Longjun Xu. 2019. "Preparation, Characterization, and Performance Analysis of S-Doped Bi2MoO6 Nanosheets" Nanomaterials 9, no. 9: 1341. https://doi.org/10.3390/nano9091341




