Millimeter-Wave-Based Spoof Localized Surface Plasmonic Resonator for Sensing Glucose Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials and Glucose-Solution Sample
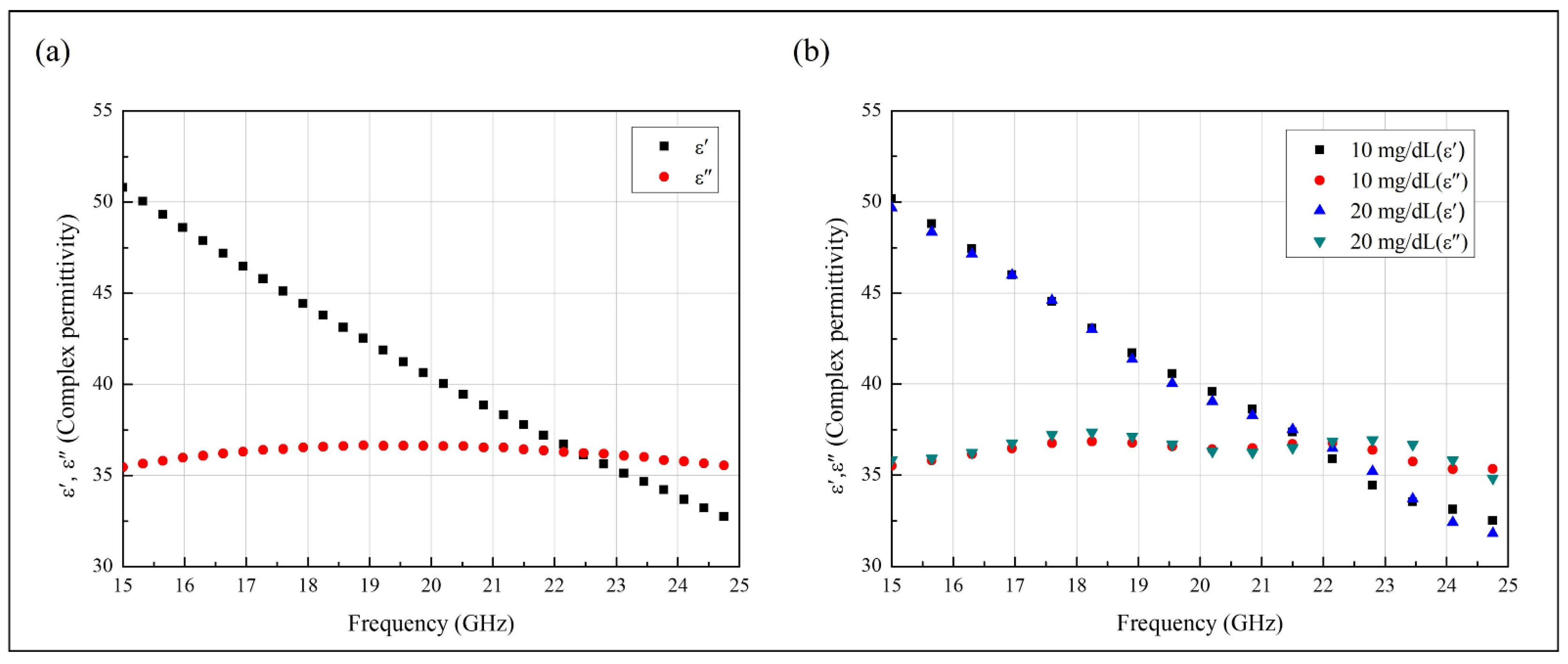
2.2. Complex Permittivity of the Solution Samples
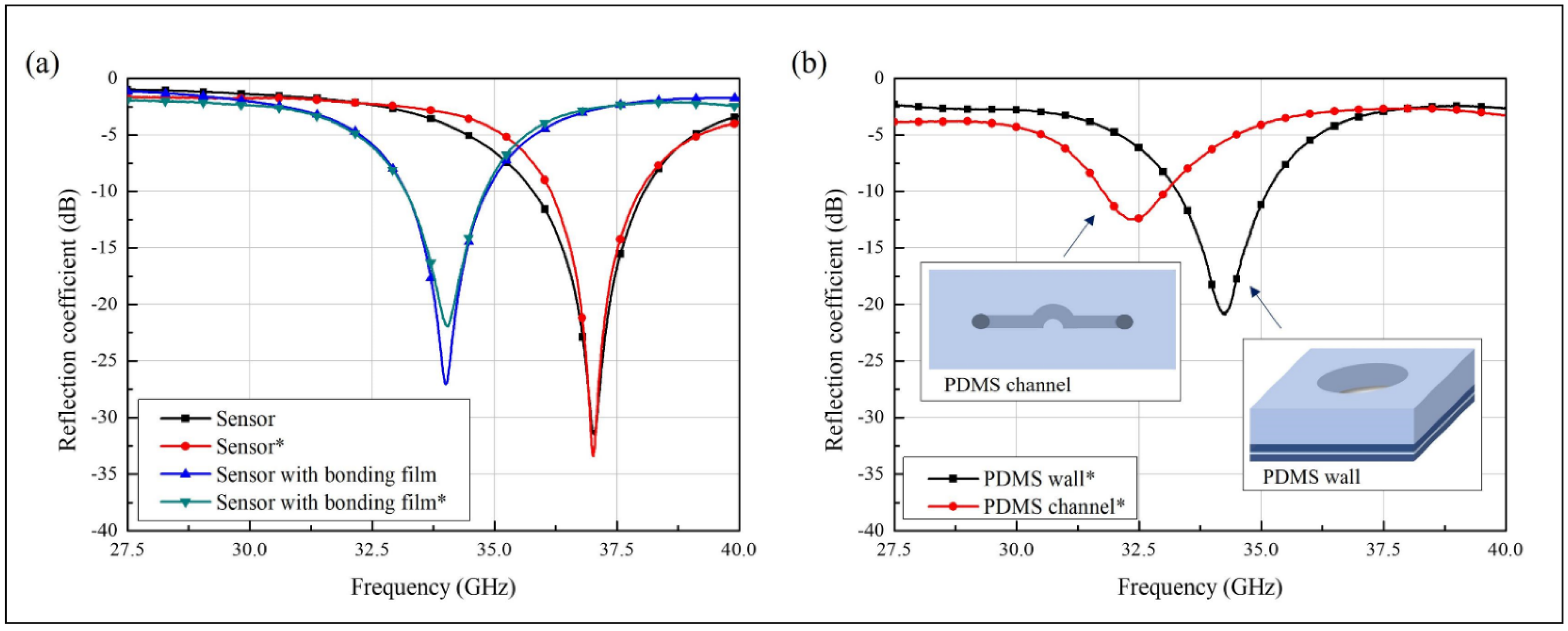
2.3. Sensor Design Based on the Spoof LSP Resonator
3. Results and Discussion
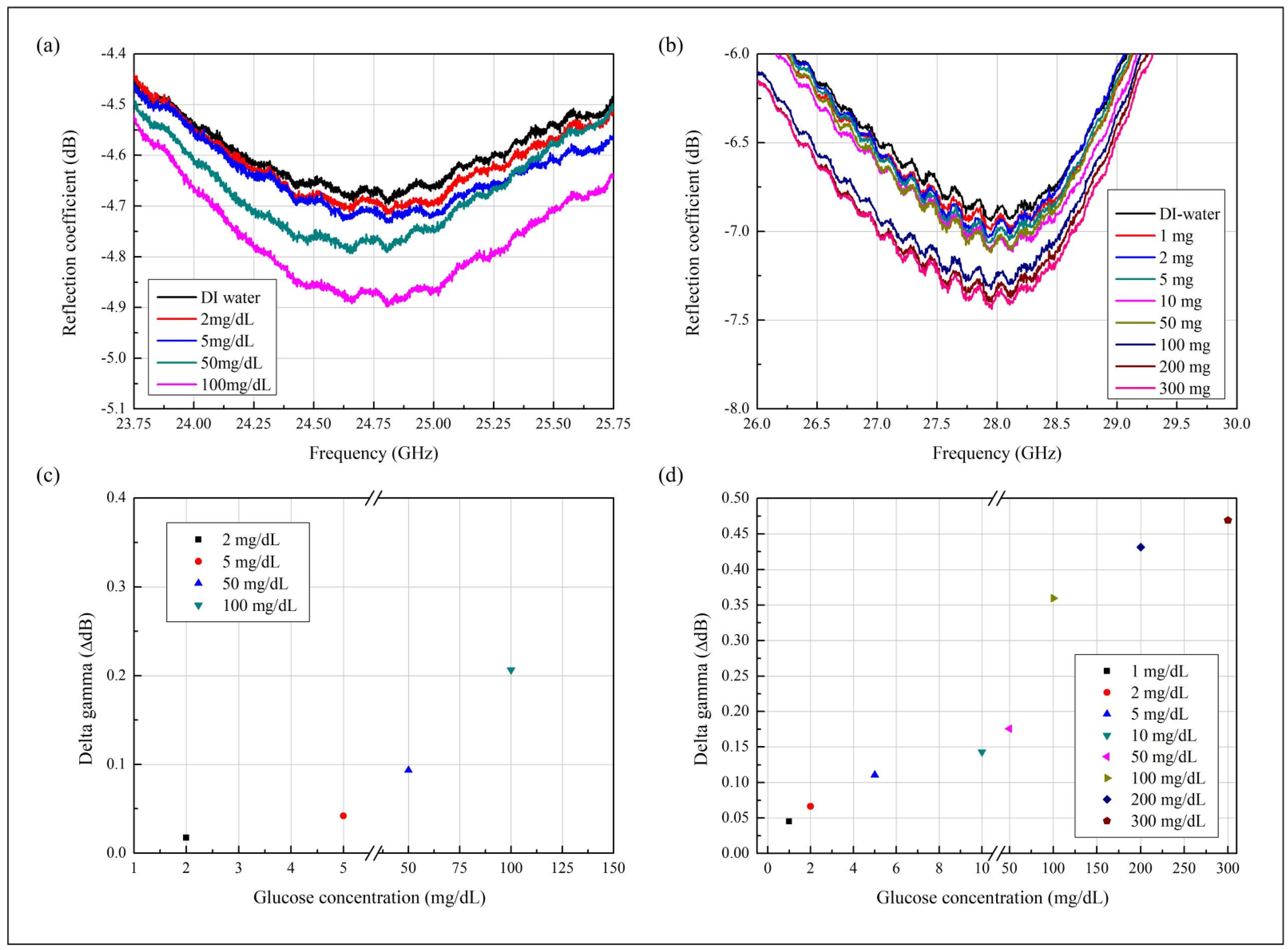
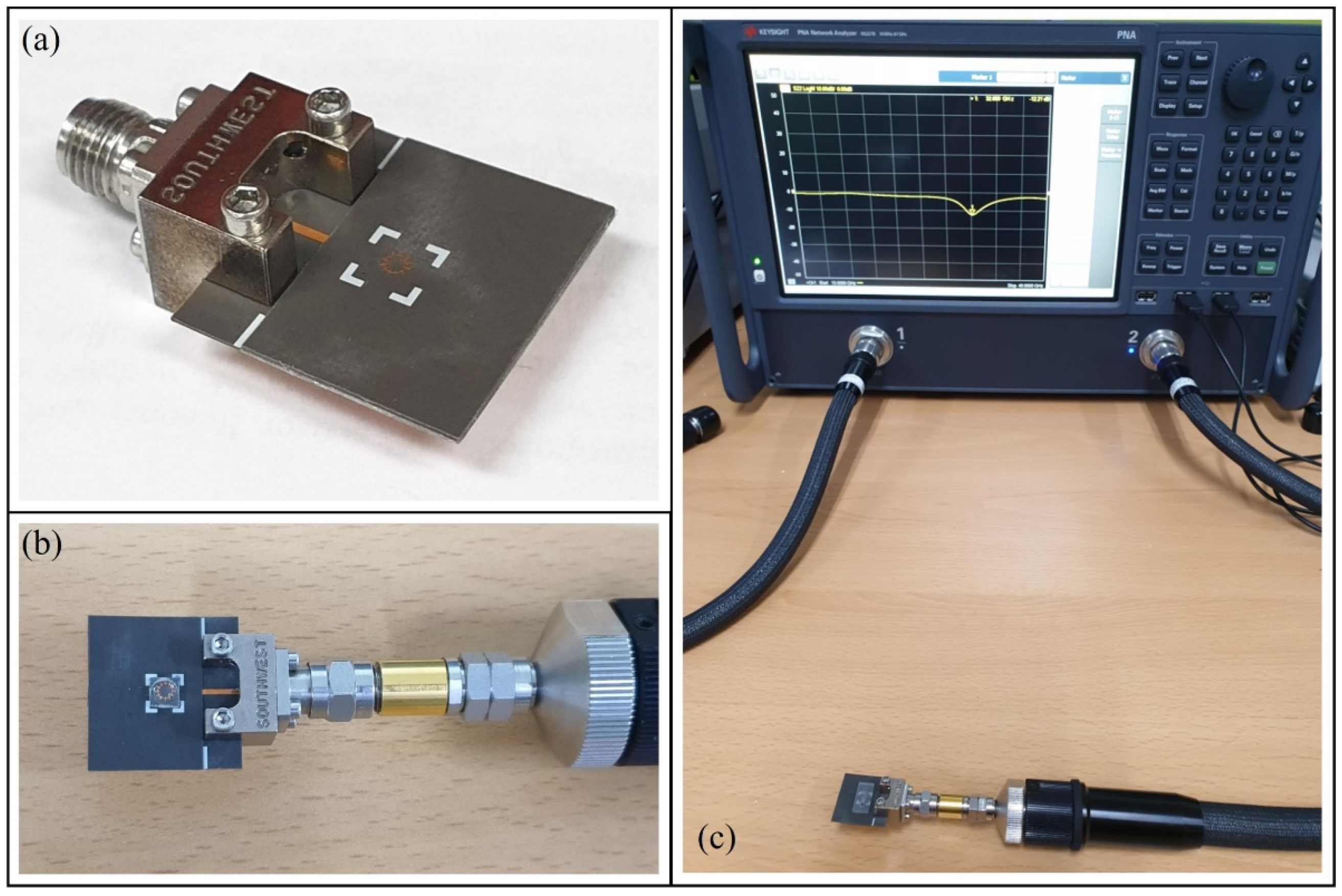
3.1. Measurement Results
3.2. Sensitivity of the Proposed Glucose Sensor
3.3. Analytical Characterization of the Sensor
3.4. Performance Comparison of the Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.Y.; Lu, M.S.C. CMOS thermal sensor arrays for enzymatic glucose detection. IEEE Sens. J. 2011, 11, 3469–3475. [Google Scholar] [CrossRef]
- Cano, L.; Guti, J.; Mayoral, C.P.; Eduardo, L.P.; Pina, S.; Carrillo, L.T. Fiber optic sensors: A review for glucose measurement. Biosensors 2021, 11, 1–19. [Google Scholar]
- Saptari, V.; Youcef-Toumi, K. Design of a mechanical-tunable filter spectrometer for noninvasive glucose measurement. Appl. Opt. 2004, 43, 2680–2688. [Google Scholar] [CrossRef]
- Chretiennot, T.; Dubuc, D.; Grenier, K. Microwave-based microfluidic sensor for non-destructive and quantitative glucose monitoring in aqueous solution. Sensors 2016, 16, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madden, J.; Barrett, C.; Laffir, F.R.; Thompson, M.; Galvin, P.; O′ Riordan, A. On-chip glucose detection based on glucose oxidase immobilized on a platinum-modified, gold microband electrode. Biosensors 2021, 11, 249. [Google Scholar] [CrossRef]
- Gorst, A.; Zavyalova, K.; Mironchev, A. Non-invasive determination of glucose concentration using a near-field sensor. Biosensors 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Patel, P.N.; Yadav, R. An ENG resonator-based microwave sensor for the characterization of aqueous glucose. J. Phys. D Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Govind, G.; Akhtar, M.J. Design of an ELC resonator-based reusable RF microfluidic sensor for blood glucose estimation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Z.; Yue, W.; Zhang, C.; Gao, S.; Wang, C. Reusable, Non-invasive, and ultrafast radio frequency biosensor based on optimized integrated passive device fabrication process for quantitative detection of glucose levels. Sensors 2020, 20, 1565. [Google Scholar] [CrossRef] [Green Version]
- Juska, V.B.; Pemble, M.E. A Critical review of electrochemical glucose sensing: Evolution of biosensor platforms based on advanced nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z. Superlenses to overcome the diffraction limit. Nat. Mater. 2008, 7, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Janković, N.; Ilić, S.; Bengin, V.; Birgermajer, S.; Radonić, V.; Alù, A. Acoustic spoof surface plasmon polaritons for filtering, isolation and sensing. Results Phys. 2021, 28, 104645. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Erratum: Plasmonics for improved photovoltaic devices (Nature Materials (2010) 9 (205–213)). Nat. Mater. 2010, 9, 865. [Google Scholar] [CrossRef] [Green Version]
- Khamsalee, P.; Mesawad, P.; Wongsan, R. Hybrid metamaterial for the secondary radar antenna system. J. Electromagn. Eng. Sci. 2020, 20, 221–233. [Google Scholar] [CrossRef]
- Pandit, N.; Jaiswal, R.K.; Pathak, N.P. Plasmonic metamaterial-based label-free microfluidic microwave sensor for aqueous biological applications. IEEE Sens. J. 2020, 20, 10582–10590. [Google Scholar] [CrossRef]
- Farokhipour, E.; Mehrabi, M.; Komjani, N.; Ding, C. A spoof surface plasmon polaritons (SSPPs) based dual-band-rejection filter with wide rejection bandwidth. Sensors 2020, 20, 7311. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Cai, J.; Jiang, J.H. Amplification of spoof localized surface plasmons on active plasmonic metamaterials. J. Phys. D Appl. Phys. 2018, 51, 295304. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, F.; Xu, H.; Zhang, Y.; Zhang, B. Localized spoof surface plasmons in textured open metal surfaces. Opt. Lett. 2016, 41, 2181. [Google Scholar] [CrossRef]
- Kianinejad, A.; Chen, Z.N.; Qiu, C.W. Design and modeling of spoof surface plasmon modes-based microwave slow-wave transmission line. IEEE Trans. Microw. Theory Tech. 2015, 63, 1817–1825. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.Y.; Lei, Y.; Zheng, X.; Zhang, J.; Cui, T.J. Spoof localized surface plasmons for sensing applications. Adv. Mater. Technol. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Koutsoupidou, M.; Cano-Garcia, H.; Pricci, R.L.; Saha, S.C.; Palikaras, G.; Kallos, E.; Kosmas, P. Study and suppression of multipath signals in a non-invasive millimeter wave transmission glucose-sensing system. IEEE J. Electromagn. RF Microw. Med. Biol. 2020, 4, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Topfer, F.; Oberhammer, J. Millimeter-waves tissue diagnostics: The most promising fields for medical applications. IEEE Microw. Mag. 2015, 16, 97–113. [Google Scholar] [CrossRef]
- Jang, C.; Park, J.K.; Lee, H.J.; Yun, G.H.; Yook, J.G. Temperature-corrected fluidic glucose sensor based on microwave resonator. Sensors 2018, 18, 3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, R.S.; Park, S.I.; Arya, A.K.; Kim, S. Continuous characterization of permittivity over a wide bandwidth using a cavity resonator. J. Electromagn. Eng. Sci. 2020, 20, 39–44. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, J.W.; Kwon, J.Y.; Kang, N.W. Characteristics of a cutoff cavity probe applicable to crack detection using the forced resonance microwave method. J. Electromagn. Eng. Sci. 2020, 20, 285–292. [Google Scholar] [CrossRef]
- Andryieuski, A.; Kuznetsova, S.M.; Zhukovsky, S.V.; Kivshar, Y.S.; Lavrinenko, A.V. Water: Promising opportunities for tunable all-dielectric electromagnetic metamaterials. Sci. Rep. 2015, 5, 13535. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.J.; Zhou, Y.J.; Xiao, Q.X. Spoof localized surface plasmons in corrugated ring structures excited by microstrip line. Opt. Express 2015, 23, 21434. [Google Scholar] [CrossRef]
- Yin, J.Y.; Ren, J.; Zhang, H.C.; Zhang, Q.; Cui, T.J. Capacitive-coupled series spoof surface plasmon polaritons. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Memon, M.U.; Lim, S. Millimeter-wave chemical sensor using substrate-integrated-waveguide cavity. Sensors 2016, 16, 1829. [Google Scholar] [CrossRef] [Green Version]
- Bao, D.; Rajab, K.Z.; Jiang, W.X.; Cheng, Q.; Liao, Z.; Cui, T.J. Experimental demonstration of compact spoof localized surface plasmons. Opt. Lett. 2016, 41, 5418. [Google Scholar] [CrossRef]
- Mehrotra, P.; Chatterjee, B.; Sen, S. EM-Wave Biosensors: A Review of RF, microwave, mm-wave and optical sensing. Sensors 2019, 19, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, R.L.; Zhou, Y.J.; Yang, L. Quarter-mode spoof plasmonic resonator for a microfluidic chemical sensor. Appl. Opt. 2018, 57, 8472. [Google Scholar] [CrossRef]
- Gholamian, M.; Shabanpour, J.; Cheldavi, A. Highly sensitive quarter-mode spoof localized plasmonic resonator for dual-detection rf microfluidic chemical sensor. J. Phys. D Appl. Phys. 2020, 53, 145401. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Zhou, Y.J.; Cai, J.; Li, Q.Y.; Li, Z.; Xiao, Z.Y. Ultra-high resolution sensing of glucose concentration based on amplified half-integer localized surface plasmons mode. J. Phys. D Appl. Phys. 2020, 53, 095305. [Google Scholar] [CrossRef]
- Kandwal, A.; Nie, Z.; Igbe, T.; Li, J.; Liu, Y.; Liu, L.W.Y.; Hao, Y. Surface plasmonic feature microwave sensor with highly confined fields for aqueous-glucose and blood-glucose measurements. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Abedeen, Z.; Agarwal, P. Microwave sensing technique based label-free and real-time planar glucose analyzer fabricated on FR4. Sens. Actuators A Phys. 2018, 279, 132–139. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Coplanar waveguide transmission line loaded with electric-LC resonator for determination of glucose concentration sensing. IEEE Sens. J. 2017, 17, 1635–1640. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Odabashyan, L.; Babajanyan, A.; Baghdasaryan, Z.; Kim, S.; Kim, J.; Friedman, B.; Lee, J.H.; Lee, K. Real-time noninvasive measurement of glucose concentration using a modified hilbert shaped microwave sensor. Sensors 2019, 19, 5525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, W.T.; Issa, K.; Ashraf, M.A.; Alshebeili, S. In vitro analysis of a microwave sensor for noninvasive glucose monitoring. Microw. Opt. Technol. Lett. 2019, 61, 599–604. [Google Scholar] [CrossRef]
- Omer, A.E.; Gigoyan, S.; Shaker, G.; Safavi-Naeini, S. WGM-based sensing of characterized glucose-aqueous solutions at mm-waves. IEEE Access 2020, 8, 38809–38825. [Google Scholar] [CrossRef]
- Hosseini, N.; Baghelani, M. Selective Real-time non-contact multi-variable water-alcohol-sugar concentration analysis during fermentation process using microwave split-ring resonator based sensor. Sens. Actuators A Phys. 2021, 325, 112695. [Google Scholar] [CrossRef]
- Characterization, M.D.; Ebrahimi, A.; Member, S.; Withayachumnankul, W. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterization. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar]



| Title [Ref. No] | Operating Frequency (GHz) | Physical Size (mm2) | Sample Volume (𝛍L) | Q-Factor 1 | Distinguishable Concentration (mg/dL) | Sensitivity | |
|---|---|---|---|---|---|---|---|
| (dB/(g/mL)) | (MHz/(g/mL)) | ||||||
| This work | 28 | 20 × 20 | 3.4 | 308.5 | 1 | 1566.9 2 | N/A |
| [15] | 3.16−3.76 3 | 20 4 × 20 4 | 101.7 | N/A | 1164 | N/A | 773 |
| [32] | 6.86−7.8 | 17 × 17 | 3.9 | 25 | N/A | 940 MHz shift (10 to 90% ethanol) | |
| [33] | 6.67 | 34 × 34 | 6 & 15.5 | N/A | N/A | detection two chemicals | |
| [34] | 1.5−2.5 | 42 × 40 | 12 | 40,000 | 9 | N/A | 29,111.1 |
| [35] | 8−12 | 52 × 24 | N/A | N/A | 25 | N/A | 200,000 |
| Title [Ref. No] | Sensing Technique | Operating Frequency (GHz) | Physical Size (mm2) | Sample Volume (𝛍L) | Q-Factor | Sensitivity | |
|---|---|---|---|---|---|---|---|
| (dB/(g/mL)) | (MHz/(g/mL)) | ||||||
| This work | Spoof LSP resonance | 28 | 20 × 20 | 3.4 | 308.5 | 1566.9 | N/A |
| [23] | CSRR | 2.9 | 26 × 40 | N/A | N/A | 7.5 | N/A |
| [36] | CPW with IDT | 3.9−4.12 2 | 25.4 × 30 | 15 | 20 1 | 15.3 | 235.32 |
| [37] | CPW with ELC | 3.41 | 16 3 × 16 3 | 20 | N/A | 3.73 | N/A |
| [38] | CSRR driven by ISM radar | 2.45 | 20 × 66 | 600 | 60 1 | N/A | 125,000 |
| [39] | Hilbert curve | 6 | 20.4 × 40.4 | 500 | 62 | 1560 | N/A |
| [40] | Microstrip | 5.5−6.7 | 80 × 80 | 14,000 | 81 1 | N/A | 54,000 |
| [41] | WGM 4 | 50−70 | 50 × 7.64 3 | 50–370 | N/A | 1000 | N/A |
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Salim, A.; Lim, S. Millimeter-Wave-Based Spoof Localized Surface Plasmonic Resonator for Sensing Glucose Concentration. Biosensors 2021, 11, 358. https://doi.org/10.3390/bios11100358
Kim Y, Salim A, Lim S. Millimeter-Wave-Based Spoof Localized Surface Plasmonic Resonator for Sensing Glucose Concentration. Biosensors. 2021; 11(10):358. https://doi.org/10.3390/bios11100358
Chicago/Turabian StyleKim, Yelim, Ahmed Salim, and Sungjoon Lim. 2021. "Millimeter-Wave-Based Spoof Localized Surface Plasmonic Resonator for Sensing Glucose Concentration" Biosensors 11, no. 10: 358. https://doi.org/10.3390/bios11100358





