A Single-Substrate Biosensor with Spin-Coated Liquid Crystal Film for Simple, Sensitive and Label-Free Protein Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formation of DMOAP Monolayer
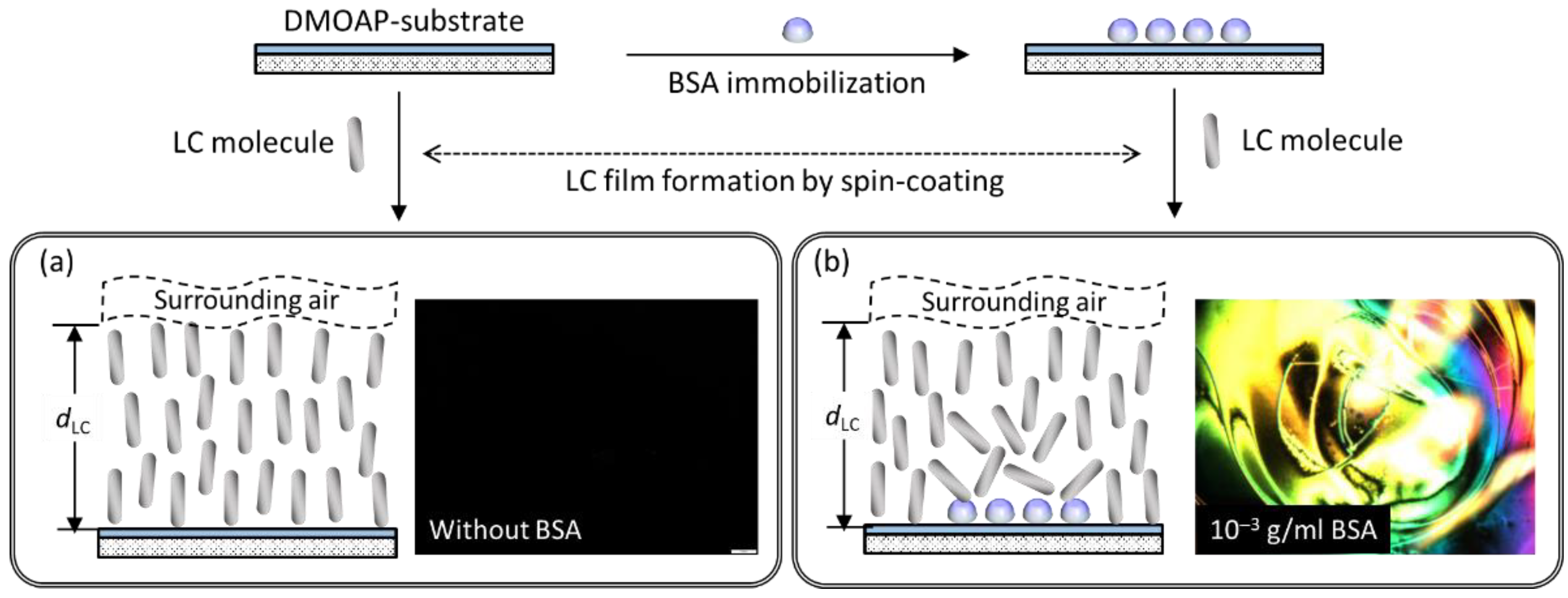
2.3. Immobilization of BSA
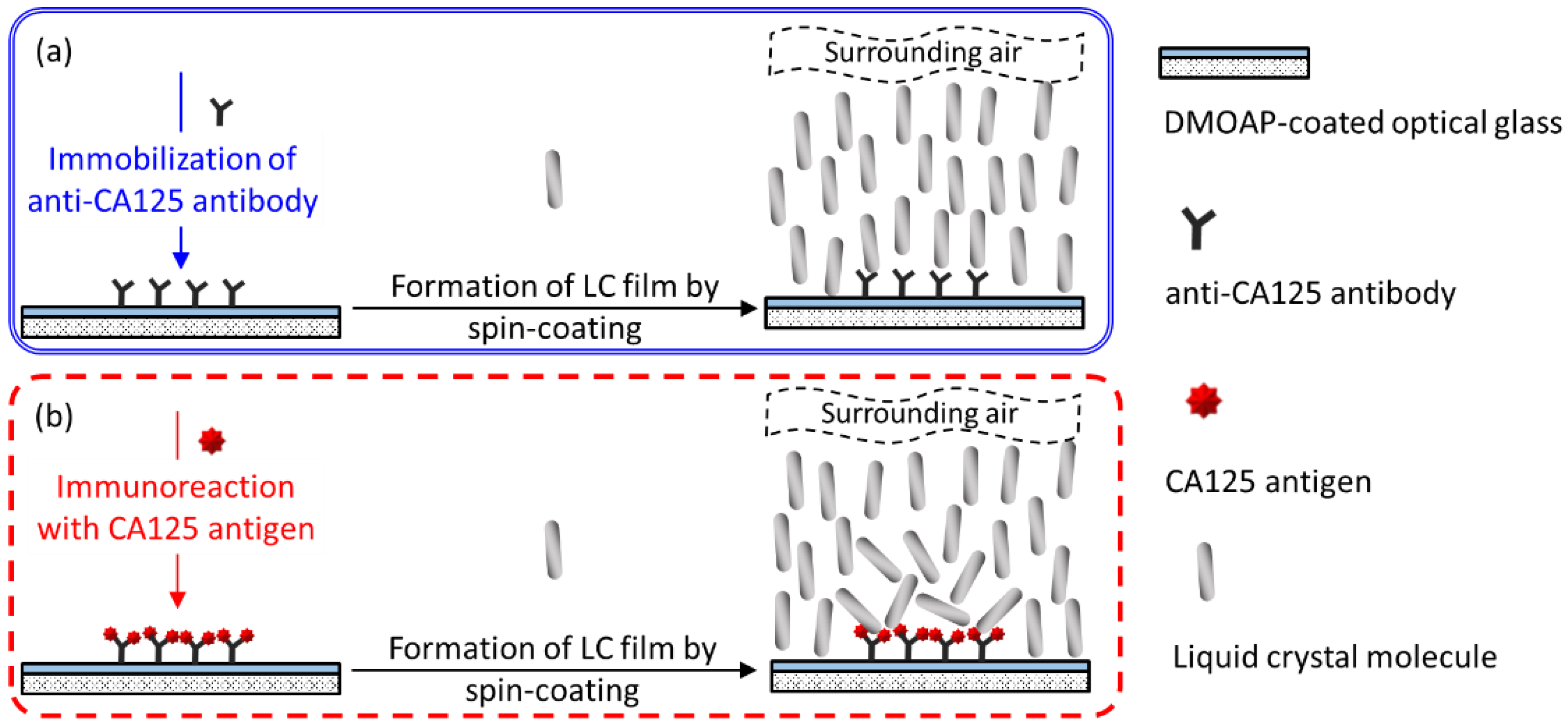
2.4. Specific Binding of Anti-CA125 Antibody to the CA125 Antigen
2.5. LC Cell Assembly and Spin-Coating of LC Films
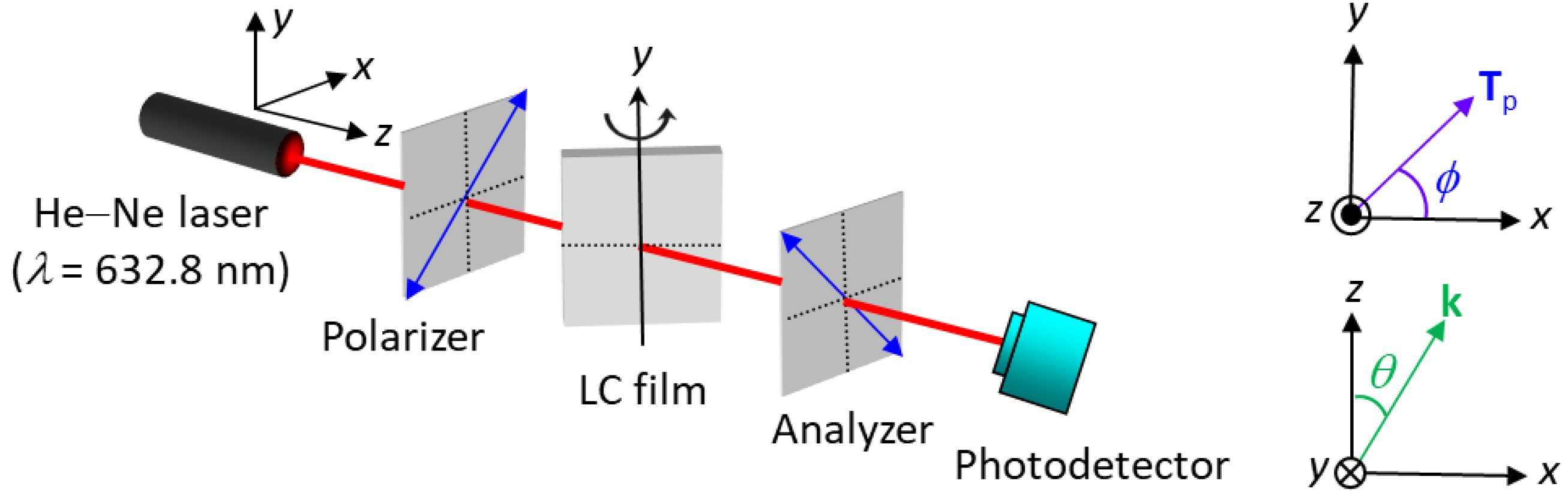
2.6. Optical LC Texture Observation and Measurement of LC Film Thickness
3. Results and Discussion
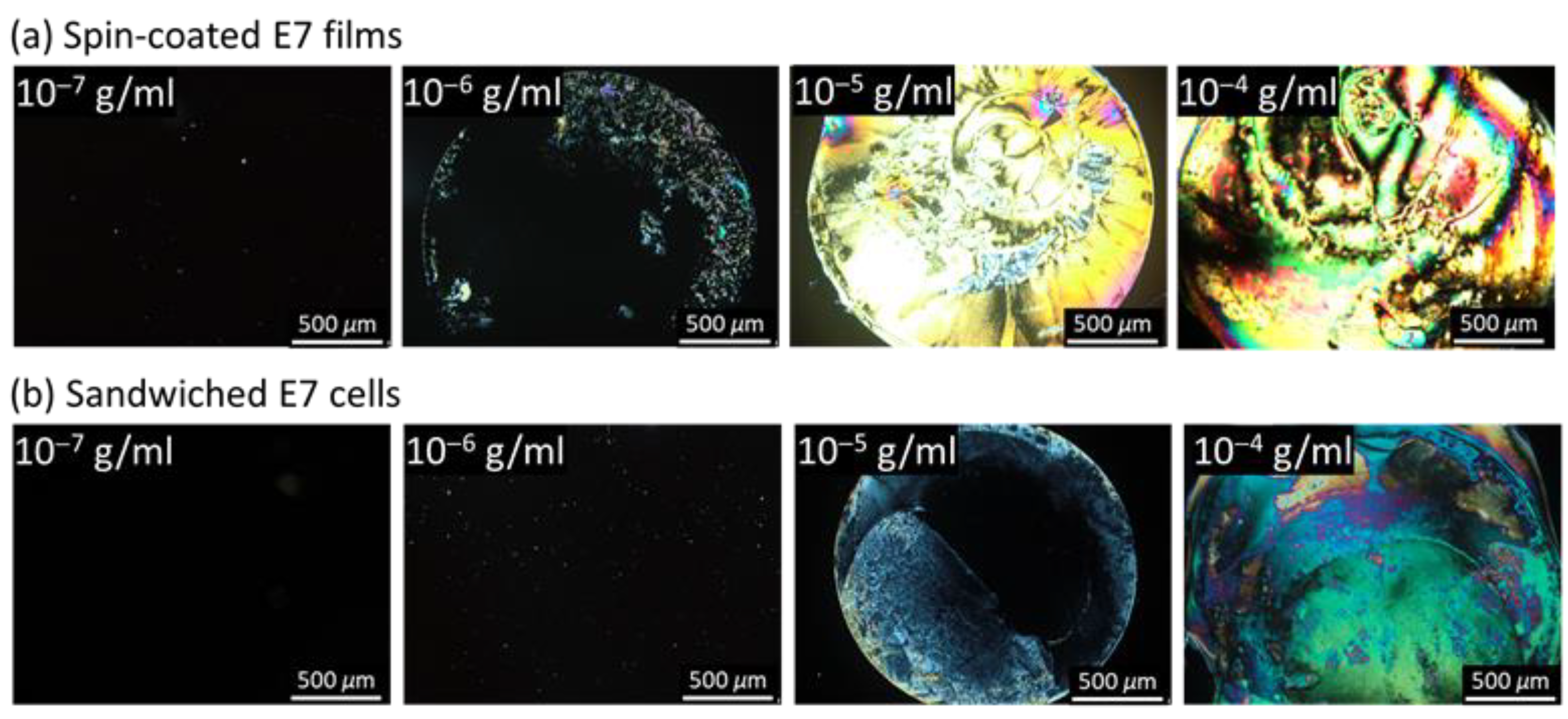
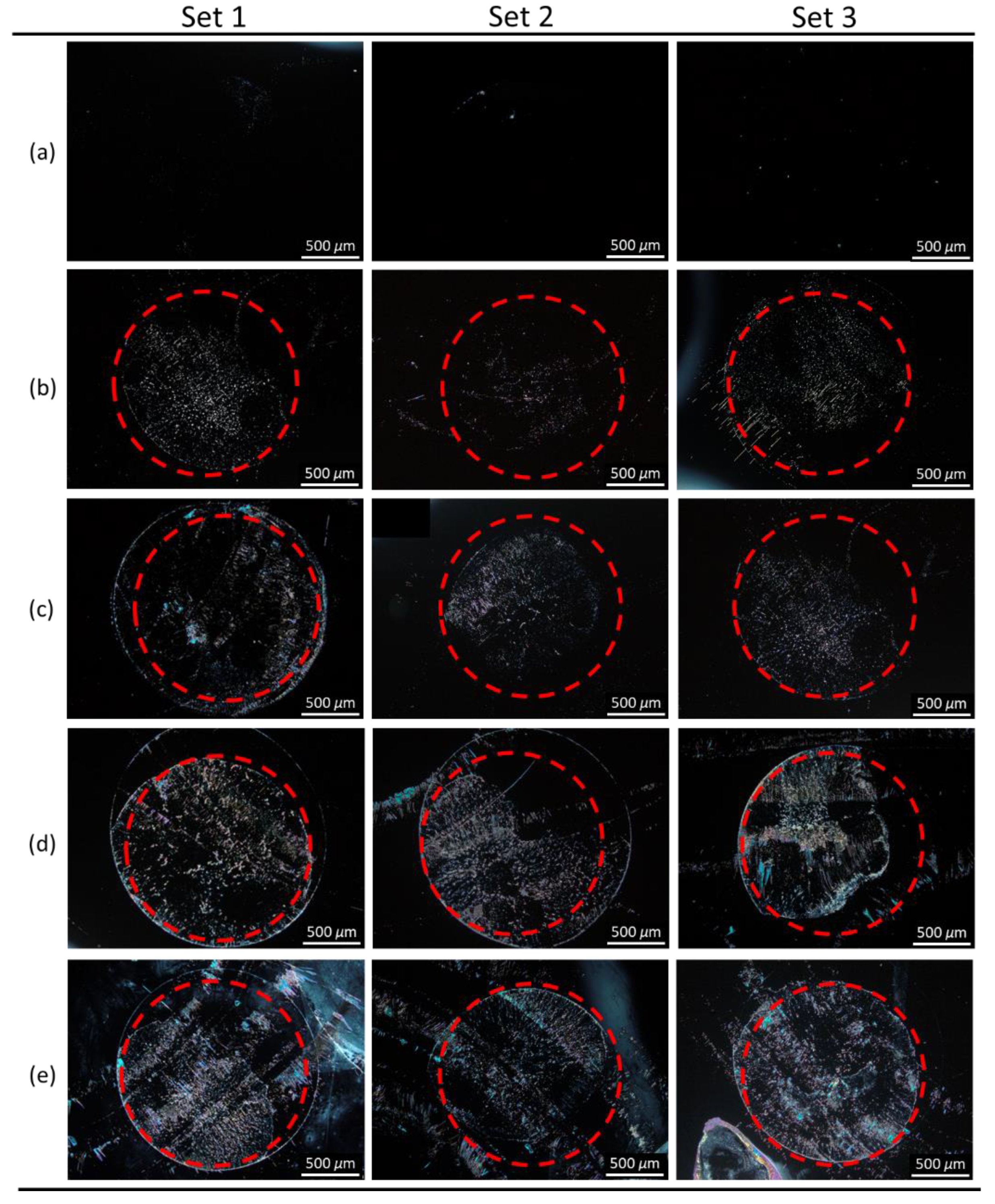
3.1. LC-Based Single-Substrate Protein Assay with Spin-Coated E7 Film
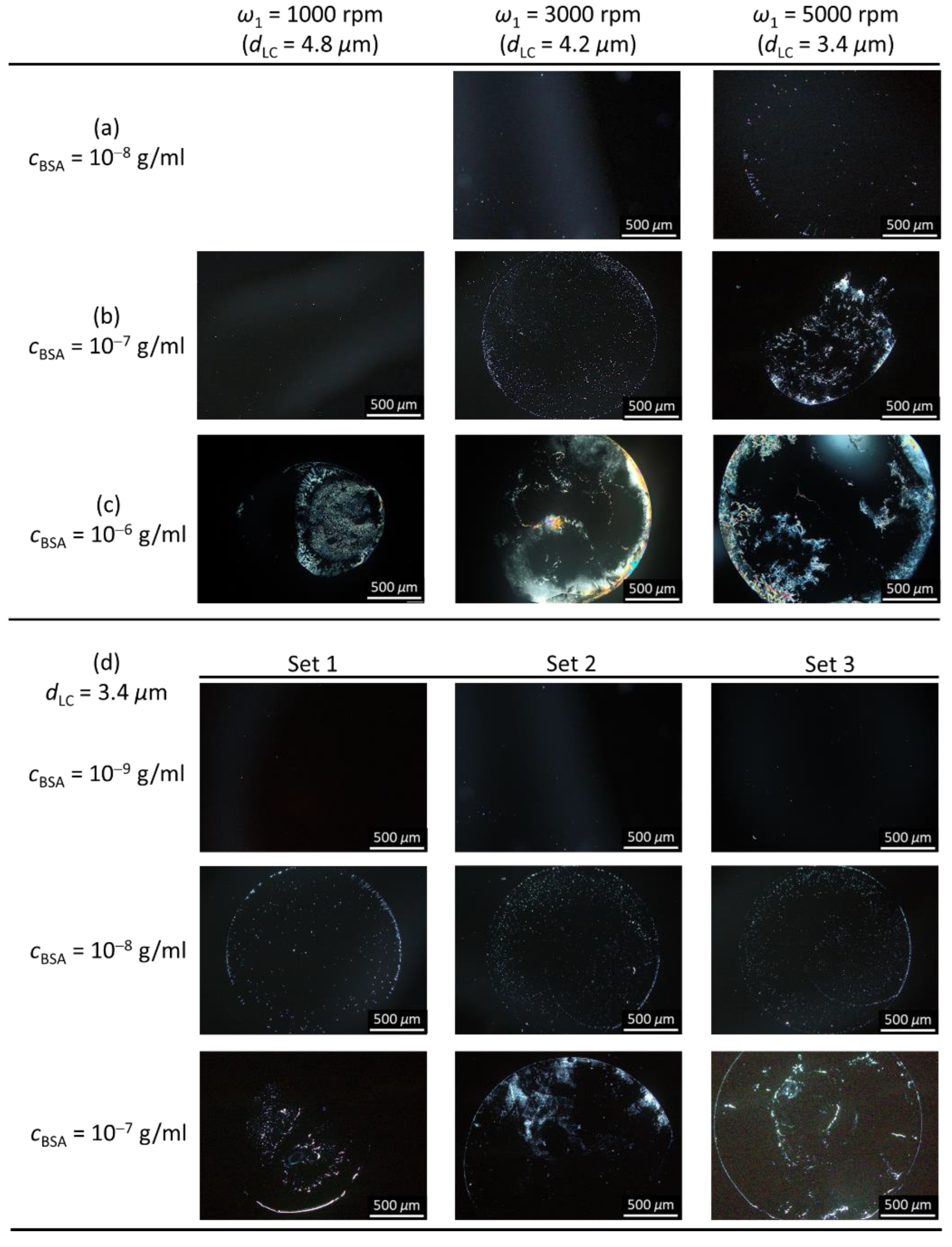
3.2. Signal Amplification of Single-Substrate Detection through the Control of Film Thickness
3.3. LC-Based Single-Substrate Immunoassay for CA125 with Spin-Coated E7 or HDN Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, V.K.; Skaife, J.J.; Dubrovsky, T.B.; Abbott, N.L. Optical amplification of ligand-receptor binding using liquid crystals. Science 1998, 279, 2077–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.S.; Carlton, R.J.; Mushenheim, P.C.; Abbott, N.L. Introduction to optical methods for characterizing liquid crystals at interfaces. Langmuir 2013, 29, 3154–3169. [Google Scholar] [CrossRef] [Green Version]
- Luan, C.; Luan, H.; Luo, D. Application and technique of liquid crystal-based biosensors. Micromachines 2020, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.-C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702. [Google Scholar] [CrossRef]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef]
- Hussain, Z.; Qazi, F.; Ahmed, M.I.; Usman, A.; Riaz, A.; Abbasi, A.D. Liquid crystals based sensing platform-technological aspects. Biosens. Bioelectron. 2016, 85, 110–127. [Google Scholar] [CrossRef]
- Prakash, J.; Parveen, A.; Mishra, Y.K.; Kaushik, A.K. Nanotechnology-assisted liquid crystals-based biosensors: Towards fundamental to advanced applications. Biosens. Bioelectron. 2020, 112562. [Google Scholar] [CrossRef]
- Popov, N.; Honaker, L.W.; Popova, M.; Usol’tseva, N.; Mann, E.K.; Jákli, A.; Popov, P. Thermotropic liquid crystal-assisted chemical and biological sensors. Materials 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lee, M.-J.; Lee, W. Bovine serum albumin detection and quantitation based on capacitance measurements of liquid crystals. Appl. Phys. Lett. 2016, 109, 093703. [Google Scholar] [CrossRef]
- Lin, C.-M.; Wu, P.-C.; Lee, M.-J.; Lee, W. Label-free protein quantitation by dielectric spectroscopy of dual-frequency liquid crystal. Sens. Actuators B Chem. 2019, 282, 158–163. [Google Scholar] [CrossRef]
- Shaban, H.; Yen, S.C.; Lee, M.J.; Lee, W. Signal amplification in an optical and dielectric biosensor employing liquid crystal-photopolymer composite as the sensing medium. Biosensors 2021, 11, 81. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Sung, Y.-C.; Lee, M.-J.; Lee, W. Highly sensitive color-indicating and quantitative biosensor based on cholesteric liquid crystal. Biomed. Opt. Express 2015, 6, 5033–5038. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Chang, C.-H.; Lee, W. Label-free protein sensing by employing blue phase liquid crystal. Biomed. Opt. Express 2017, 8, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-J.; Pai, C.-P.; Wu, P.-C.; Lee, W. Label-free single-substrate quantitative protein assay based on optical characteristics of cholesteric liquid crystals. J. Mol. Liq. 2021, 331, 115756. [Google Scholar] [CrossRef]
- Chiang, Y.-L.; Lee, M.-J.; Lee, W. Enhancing detection sensitivity in quantitative protein detection based on dye-doped liquid crystals. Dyes Pigment. 2018, 157, 117–122. [Google Scholar] [CrossRef]
- Wu, P.-C.; Karn, A.; Lee, M.-J.; Lee, W.; Chen, C.-Y. Dye-liquid-crystal-based biosensing for quantitative protein assay. Dyes Pigment. 2018, 150, 73–78. [Google Scholar] [CrossRef]
- Brake, J.M.; Mezera, A.D.; Abbott, N.L. Effect of surfactant structure on the orientation of liquid crystals at aqueous—liquid crystal interfaces. Langmuir 2003, 19, 6436–6442. [Google Scholar] [CrossRef]
- Hussain, Z.; Zafiu, C.; Küpcü, S.; Pivetta, L.; Hollfelder, N.; Masutani, A.; Kilickiran, P.; Sinner, E.-K. Liquid crystal based sensors monitoring lipase activity: A new rapid and sensitive method for cytotoxicity assays. Biosens. Bioelectron. 2014, 56, 210–216. [Google Scholar] [CrossRef]
- Hartono, D.; Bi, X.; Yang, K.L.; Yung, L.Y.L. An air-supported liquid crystal system for real-time and label-free characterization of phospholipases and their inhibitors. Adv. Funct. Mater. 2008, 18, 2938–2945. [Google Scholar] [CrossRef]
- Popov, P.; Honaker, L.W.; Kooijman, E.E.; Mann, E.K.; Jákli, A.I. A liquid crystal biosensor for specific detection of antigens. Sens. Bio-Sens. Res. 2016, 8, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.-Y.; Yang, K.-L. Dark-to-bright optical responses of liquid crystals supported on solid surfaces decorated with proteins. Langmuir 2008, 24, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, K.-L. Microfluidic immunoassay with plug-in liquid crystal for optical detection of antibody. Anal. Chim. Acta 2015, 853, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-J.; Chen, F.-L.; Liou, J.-C.; Huang, Y.-W.; Chen, C.-H.; Hong, Z.-Y.; Lin, J.-D.; Hsiao, Y.-C. Label-free multi-microfluidic immunoassays with liquid crystals on polydimethylsiloxane biosensing chips. Polymers 2020, 12, 395. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-W.; Hisamoto, H.; Chen, C.-H. Quantitative analysis of liquid crystal-based immunoassay using rectangular capillaries as sensing platform. Opt. Express 2019, 27, 17080–17090. [Google Scholar] [CrossRef]
- Liu, D.; Jang, C.-H. A new strategy for imaging urease activity using liquid crystal droplet patterns formed on solid surfaces. Sens. Actuators B Chem. 2014, 193, 770–773. [Google Scholar] [CrossRef]
- Han, G.-R.; Jang, C.-H. Detection of heavy-metal ions using liquid crystal droplet patterns modulated by interaction between negatively charged carboxylate and heavy-metal cations. Talanta 2014, 128, 44–50. [Google Scholar] [CrossRef]
- Dhara, P.; Mukherjee, R. Phase transition and dewetting of a 5CB liquid crystal thin film on a topographically patterned substrate. RSC Adv. 2019, 9, 21685–21694. [Google Scholar] [CrossRef]
- Dhara, P.; Bhandaru, N.; Das, A.; Mukherjee, R. Transition from spin dewetting to continuous film in spin coating of liquid crystal 5CB. Sci. Rep. 2018, 8, 7169. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.-L.; Duan, W.; Tang, M.-J.; Chen, L.-J.; Liang, X.; Lu, Y.-Q.; Hu, W. Light-driven rotation and pitch tuning of self-organized cholesteric gratings formed in a semi-free film. Polymers 2017, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Duan, F.F.; Wu, P.C.; Lee, W. Liquid crystal-photopolymer composite films for label-free single-substrate protein quantitation and immunoassay. Biomed. Opt. Express 2020, 11, 4915–4927. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Yan, J.; Ishinabe, T.; Sugiura, N.; Liu, C.-Y.; Huang, T.-H.; Tsai, C.-Y.; Lin, C.-H.; Wu, S.-T. Blue-phase liquid crystal displays with vertical field switching. J. Disp. Technol. 2012, 8, 98–103. [Google Scholar] [CrossRef]
- Liao, S.; Qiao, Y.; Han, W.; Xie, Z.; Wu, Z.; Shen, G.; Yu, R. Acetylcholinesterase liquid crystal biosensor based on modulated growth of gold nanoparticles for amplified detection of acetylcholine and inhibitor. Anal. Chem. 2012, 84, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Tan, H.; Wu, C.; Wu, Z.; Shen, G.; Yu, R. Gold nanoparticle based signal enhancement liquid crystal biosensors for DNA hybridization assays. Chem. Commun. 2012, 48, 2861–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Peng, Y.; Xu, L.; Zhou, W.; Wang, Q.; Guo, L. Liquid-crystal biosensor based on nickel-nanosphere-induced homeotropic alignment for the amplified detection of thrombin. ACS Appl. Mater. Interfaces 2015, 7, 23418–23422. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xiong, X.; Deng, S. Gold nanoparticle-based signal enhancement of an aptasensor for ractopamine using liquid crystal based optical imaging. Microchim. Acta 2019, 186, 697. [Google Scholar] [CrossRef] [PubMed]
- Nandi, R.; Loitongbam, L.; De, J.; Jain, V.; Pal, S.K. Gold nanoparticle-mediated signal amplification of liquid crystal biosensors for dopamine. Analyst 2019, 144, 1110–1114. [Google Scholar] [CrossRef]
- Su, H.-W.; Lee, Y.-H.; Lee, M.-J.; Hsu, Y.-C.; Lee, W. Label-free immunodetection of the cancer biomarker CA125 using high-Δn liquid crystals. J. Biomed. Opt. 2014, 19, 077006. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-H.; Lee, M.-J.; Lee, Y.-H.; Lee, W.; Song, X.; Chen, C.-Y. Immunoassays for the cancer biomarker CA125 based on a large-birefringence nematic liquid-crystal mixture. Biomed. Opt. Express 2015, 6, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Su, H.-W.; Lee, M.-J.; Lee, W. Surface modification of alignment layer by ultraviolet irradiation to dramatically improve the detection limit of liquid-crystal-based immunoassay for the cancer biomarker CA125. J. Biomed. Opt. 2015, 20, 057004. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Lee, M.-J.; Lee, W. Electric-field-assisted signal amplification for label-free liquid-crystal-based detection of biomolecules. Biomed. Opt. Express 2019, 10, 4987–4998. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.; Park, S.-Y. Preparation of QP4VP-b-LCP liquid crystal block copolymer and its application as a biosensor. Anal. Bioanal. Chem. 2014, 406, 5369–5378. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ren, L.; Shan, Y.; Wang, X.; Yang, Z.; Li, C.; Xu, J.; Ma, B. Smartphone-based rapid quantification of viable bacteria by single-cell microdroplet turbidity imaging. Analyst 2018, 143, 3309–3316. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-C.; Pai, C.-P.; Lee, M.-J.; Lee, W. A Single-Substrate Biosensor with Spin-Coated Liquid Crystal Film for Simple, Sensitive and Label-Free Protein Detection. Biosensors 2021, 11, 374. https://doi.org/10.3390/bios11100374
Wu P-C, Pai C-P, Lee M-J, Lee W. A Single-Substrate Biosensor with Spin-Coated Liquid Crystal Film for Simple, Sensitive and Label-Free Protein Detection. Biosensors. 2021; 11(10):374. https://doi.org/10.3390/bios11100374
Chicago/Turabian StyleWu, Po-Chang, Chao-Ping Pai, Mon-Juan Lee, and Wei Lee. 2021. "A Single-Substrate Biosensor with Spin-Coated Liquid Crystal Film for Simple, Sensitive and Label-Free Protein Detection" Biosensors 11, no. 10: 374. https://doi.org/10.3390/bios11100374




