Cocaine Detection by a Laser-Induced Immunofluorometric Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Buffers, Materials, and Equipment
2.2. Benzoylecogonine (BEC)-BSA Conjugates and Indirect Competitive Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Manufacturing of the BEC-BSA Affinity Column and Cocaine Dilutions
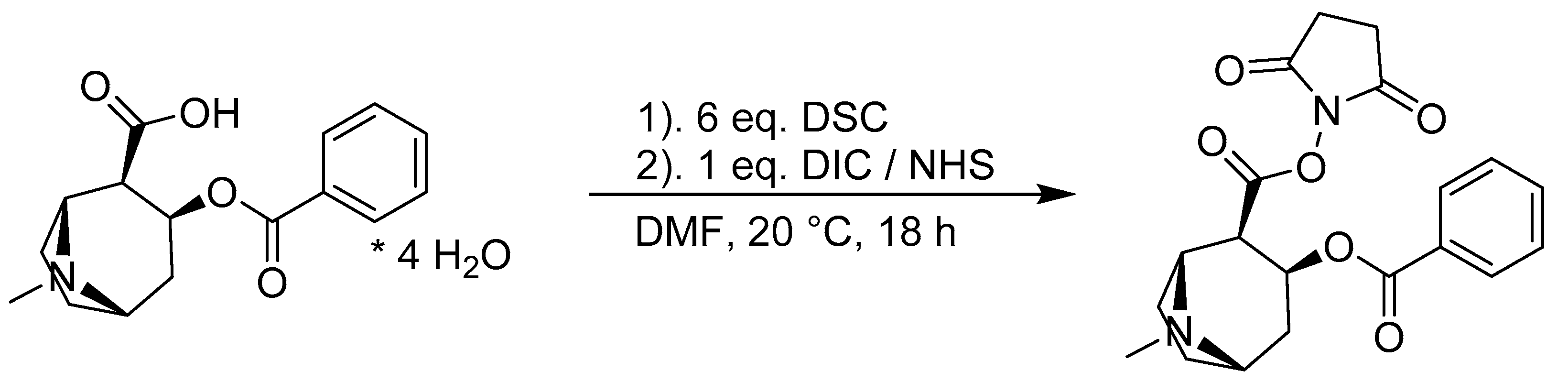
2.4. Design and Synthesis of the IP3G2 Fluorophore Conjugate
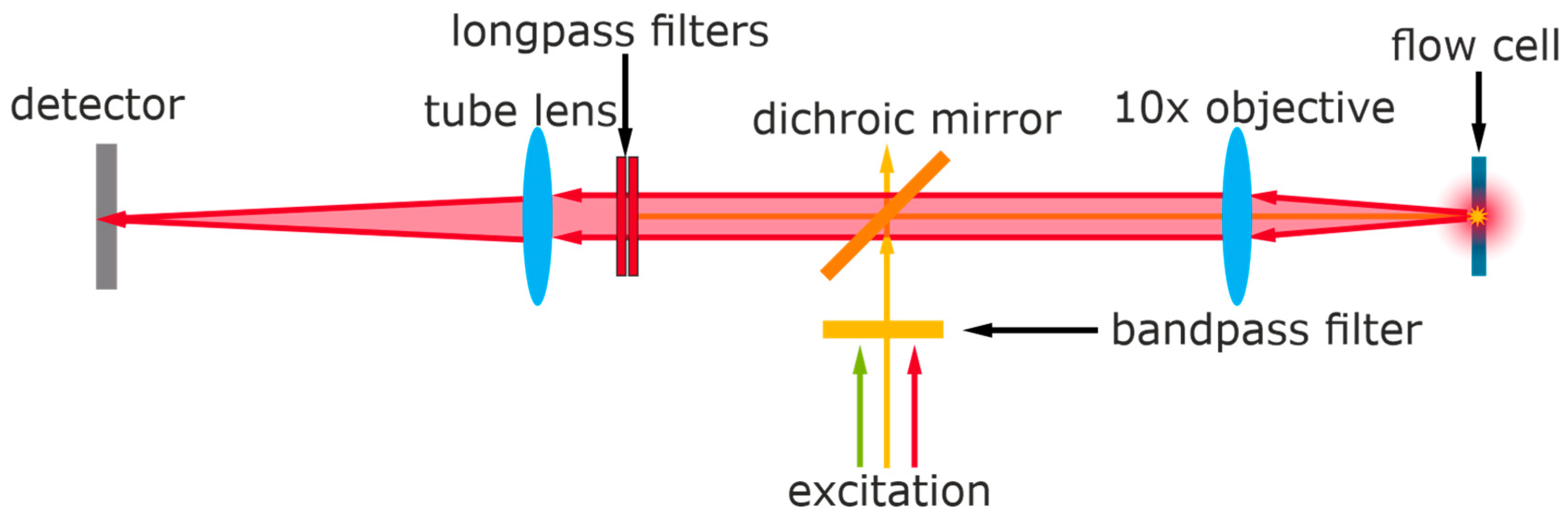
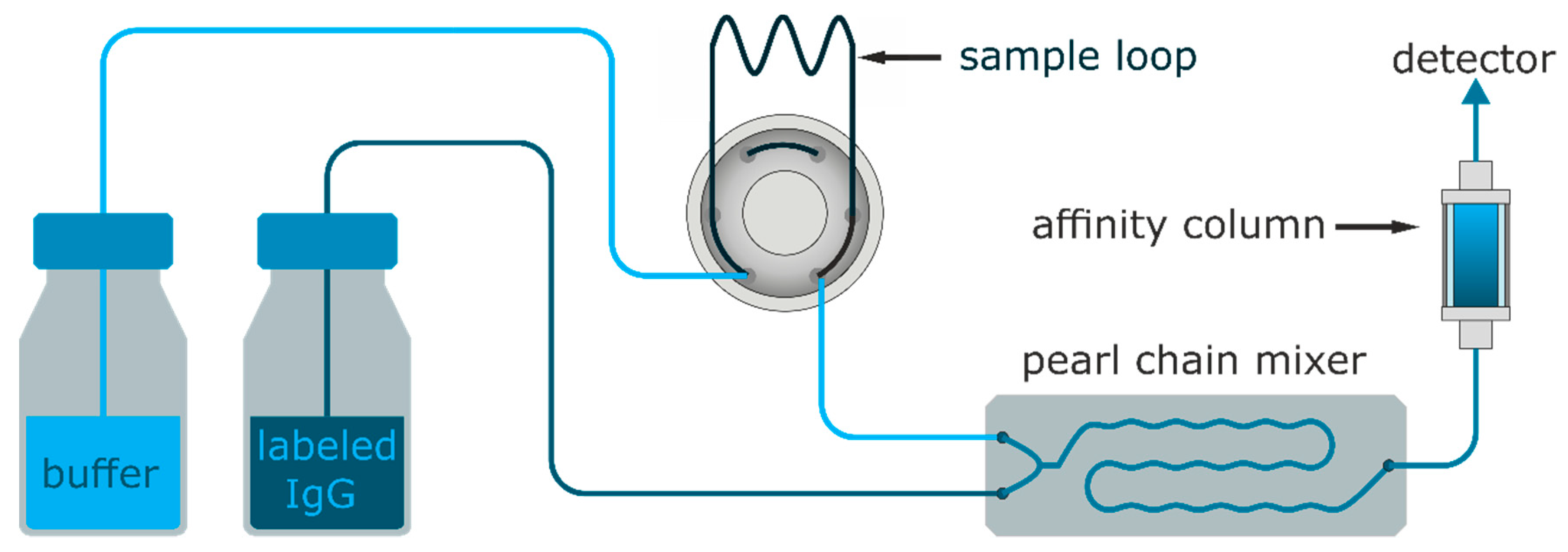
2.5. Fluorescence Detector, Fluidics, and Measurements
- (a)
- Cocaine was diluted from a stock solution to 100 to 3200 pM in 200 pM steps in PBSB. A constant flow of diluted IP3G2-Dy-654 in PBSB was mixed 1:1 with a constant flow of PBSB as described above. The injection valve was equipped with a sample loop of 200 µL, and the samples were injected every six minutes.
- (b)
- Cocaine was diluted from a stock solution to 200 to 1000 pM in 200 pM steps in PBSB. A constant flow of diluted IP3G2-Dy-654 in PBSB was mixed 1:1 with a constant flow of PBSB as described above. The injection valve was equipped with a sample loop of 500 µL, and the samples were injected in cycles of six minutes.
2.6. Data Evaluation
3. Results
3.1. BEC-BSA Synthesis and Conjugate Characterization
3.2. Antibody-Labelling and Indirect Competitive ELISA
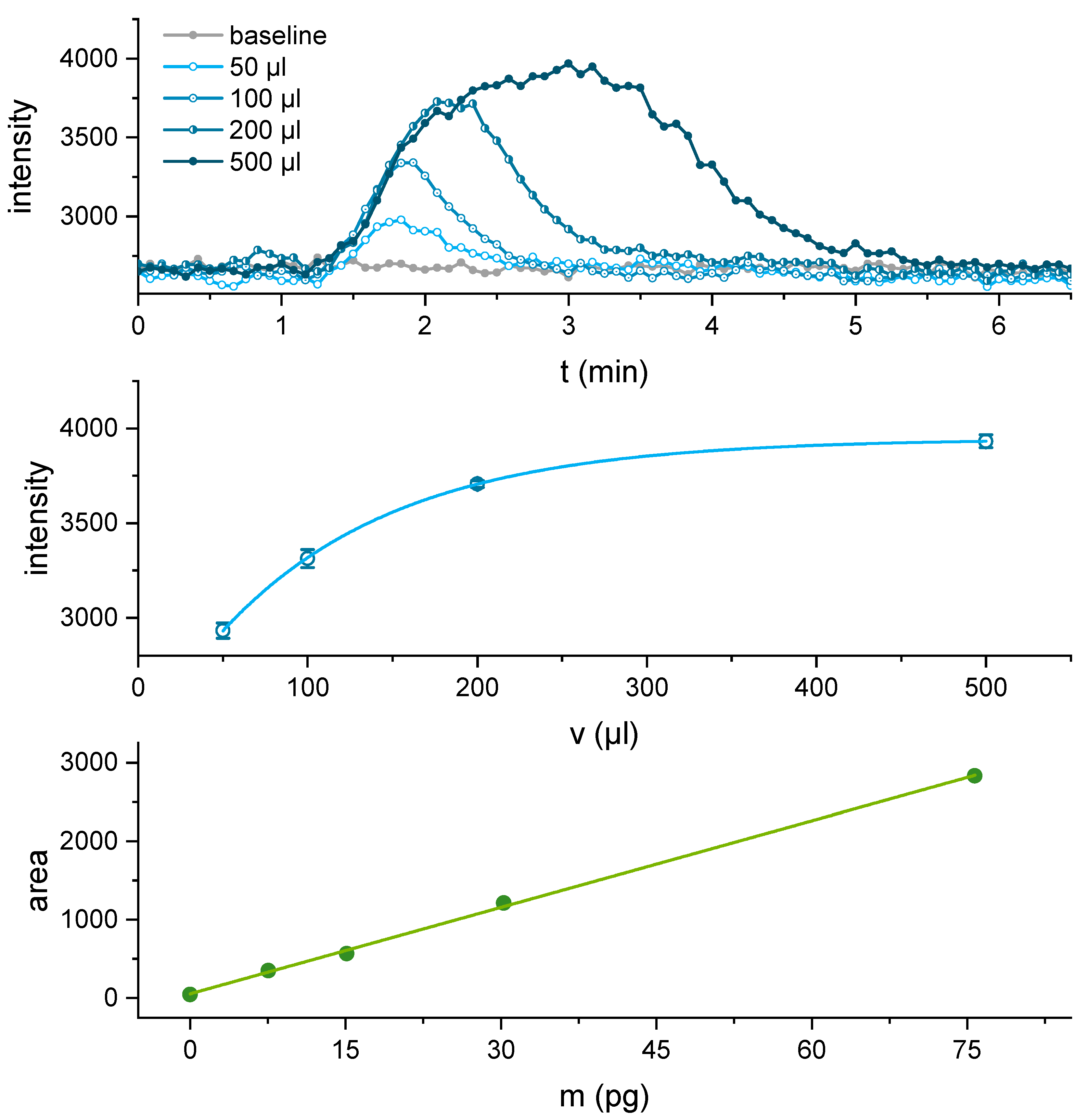
3.3. BEC-BSA Column Performance: Influence of the Injection Volume and the Reaction Time
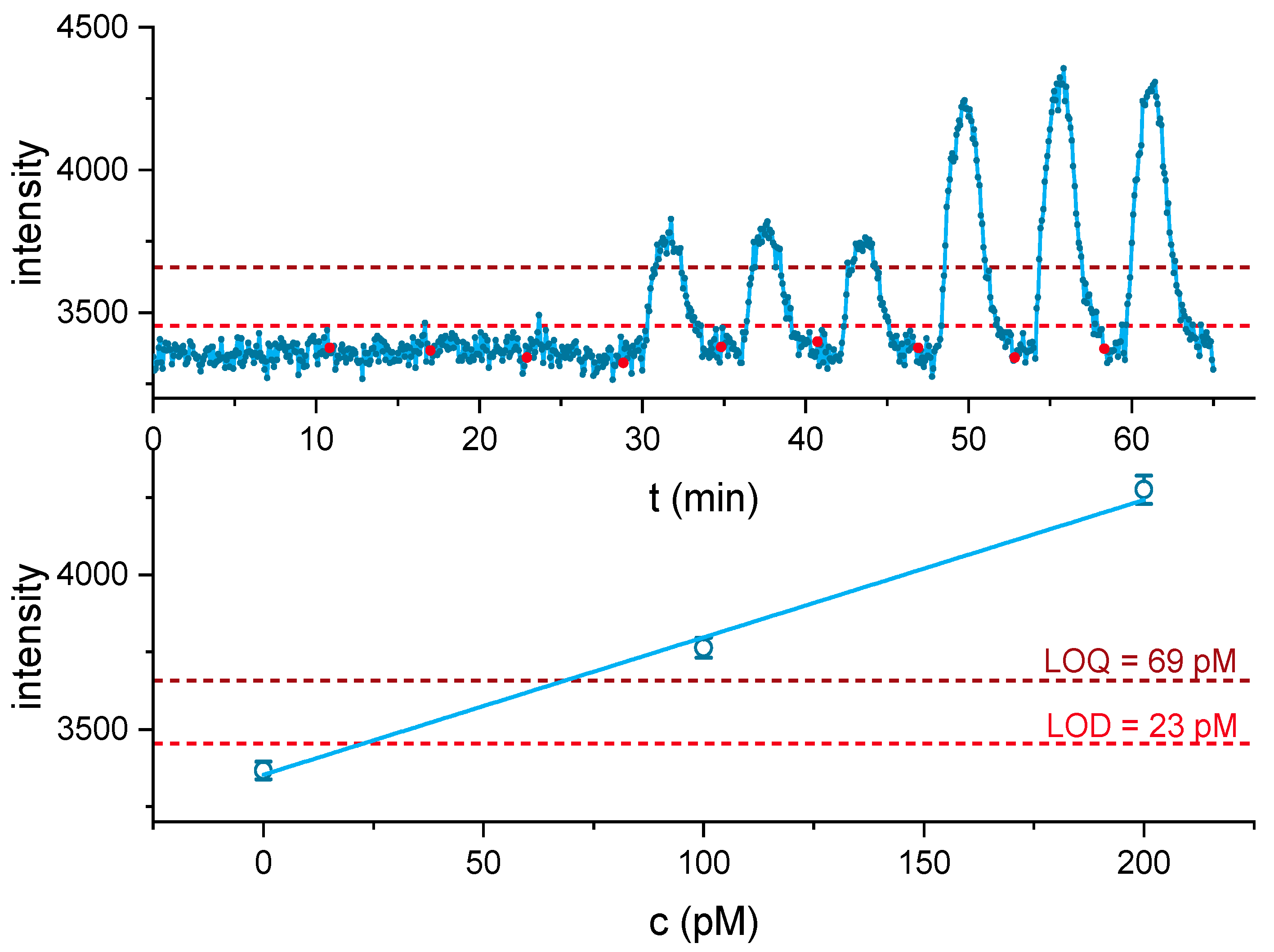
3.4. Dynamic Range and Limit of Detection for Cocaine Detection
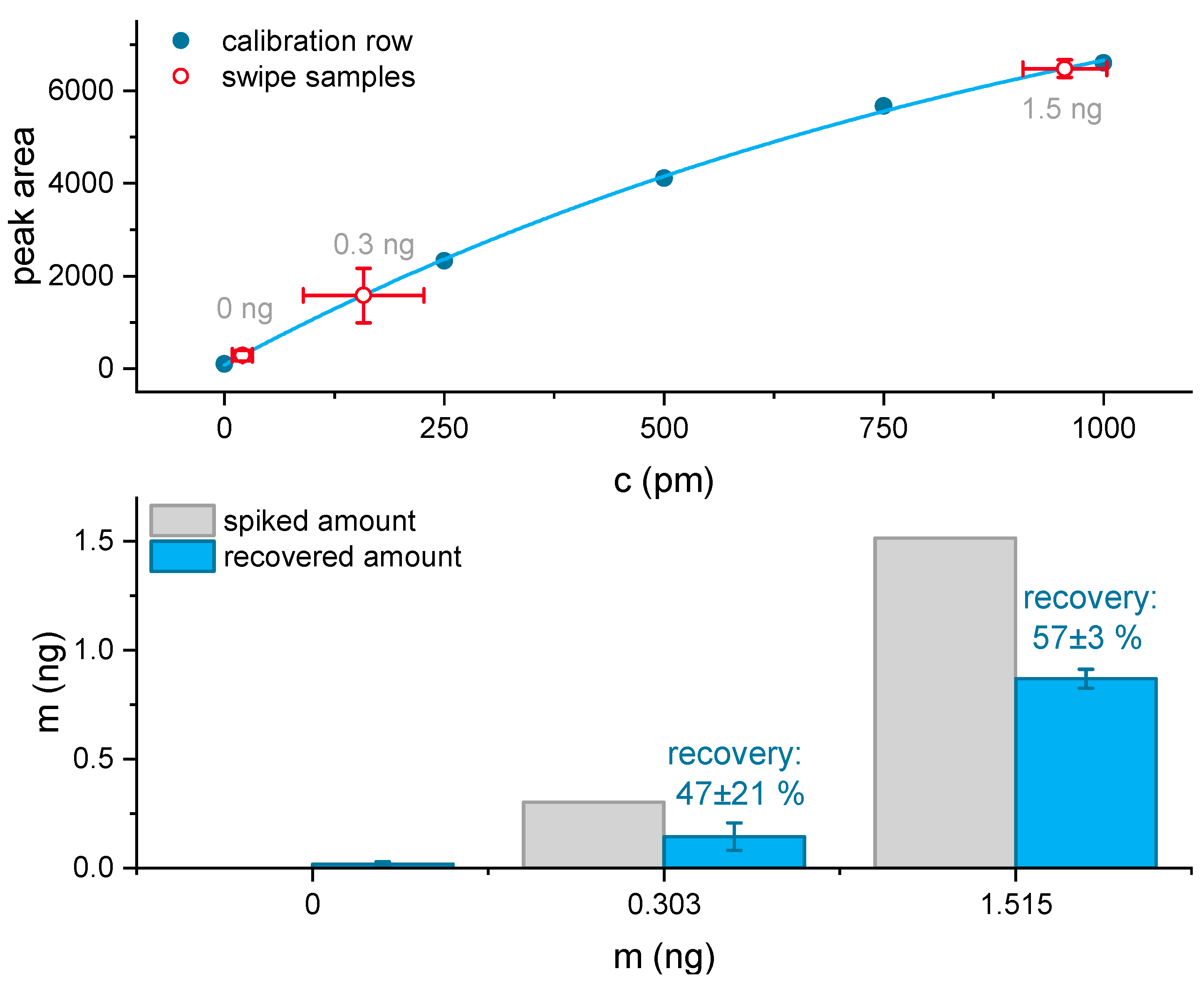
3.5. Surface Sampling, Reaction Time, and Analyte Recovery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Drug Report 2021: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction (EMCDDA): Lisbon, Portugal, 2021. [CrossRef]
- De Jong, M.; Florea, A.; Eliaerts, J.; Van Durme, F.; Samyn, N.; De Wael, K. Tackling Poor Specificity of Cocaine Color Tests by Electrochemical Strategies. Anal. Chem. 2018, 90, 6811–6819. [Google Scholar] [CrossRef] [PubMed]
- Eliaerts, J.; Meert, N.; Van Durme, F.; Dardenne, P.; Charles, S.; De Wael, K.; Samyn, N. Challenges for cocaine detection in smuggling samples. Forensic Sci. Int. 2021, 319, 110534. [Google Scholar] [CrossRef]
- Jones, N.S.; Comparin, J.H. Interpol review of controlled substances 2016–2019. Forensic Sci. Int. Synerg. 2020, 2, 608–669. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.P.; Najarro, M. Ion mobility spectrometry nuisance alarm threshold analysis for illicit narcotics based on environmental background and a ROC-curve approach. Analyst 2016, 141, 4438–4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliaro, F.; Smith, F.P.; DeBattisti, Z.; Manetto, G.; Marigo, M. Hair analysis, a novel tool in forensic and biomedical sciences: Chromatographic and electrophoretic/electrokinetic analytical strategies. J. Chromatogr. B 1997, 689, 261–271. [Google Scholar] [CrossRef]
- Selavka, C.M.; Rieders, F. The Determination of Cocaine in Hair—A Review. Forensic Sci. Int. 1995, 70, 155–164. [Google Scholar] [CrossRef]
- Musshoff, F.; Thieme, D.; Schwarz, G.; Sachs, H.; Skopp, G.; Franz, T. Determination of hydroxy metabolites of cocaine in hair samples for proof of consumption. Drug Test. Anal. 2018, 10, 681–688. [Google Scholar] [CrossRef]
- Harrison, R.; Fu, S. A Review of Methodology for Testing Hair for Cocaine. J. Forensic Investig. 2014, 2, 8. [Google Scholar]
- Hall, A.B.; Coy, S.L.; Nazarov, E.G.; Vouros, P. Rapid Separation and Characterization of Cocaine and Cocaine Cutting Agents by Differential Mobility Spectrometry-Mass Spectrometry. J. Forensic Sci. 2012, 57, 750–756. [Google Scholar] [CrossRef]
- Mieczkowski, T.; Hussey, B.; Mumm, R.; Connick, H.F. Investigating potential differences between cocaine users and distributors using the ion mobility spectrometer. J. Drug Issues 2000, 30, 147–169. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhao, Q. Direct fluorescence anisotropy assay for cocaine using tetramethylrhodamine-labeled aptamer. Anal. Bioanal. Chem. 2017, 409, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, Y.K.; Kim, O.B.; An, S.G.; Shin, M.W.; Maeng, S.J.; Choi, G.S. Comparison of Cocaine Detections in Corona Discharge Ionization-Ion Mobility Spectrometry and in Atmospheric Pressure Chemical Ionization-Mass Spectrometry. Bull. Korean Chem. Soc. 2010, 31, 2383–2385. [Google Scholar] [CrossRef] [Green Version]
- Bailey, M.J.; Bradshaw, R.; Francese, S.; Salter, T.L.; Costa, C.; Ismail, M.; Webb, R.P.; Bosman, I.; Wolff, K.; de Puit, M. Rapid detection of cocaine, benzoylecgonine and methylecgonine in fingerprints using surface mass spectrometry. Analyst 2015, 140, 6254–6259. [Google Scholar] [CrossRef] [Green Version]
- Ambach, L.; Menzies, E.; Parkin, M.C.; Kicman, A.; Archer, J.R.H.; Wood, D.M.; Dargan, P.I.; Stove, C. Quantification of cocaine and cocaine metabolites in dried blood spots from a controlled administration study using liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2019, 11, 709–720. [Google Scholar] [CrossRef]
- Huestis, M.A.; Oyler, J.M.; Cone, E.J.; Wstadik, A.T.; Schoendorfer, D.; Joseph, R.E. Sweat testing for cocaine, codeine and metabolites by gas chromatography—Mass spectrometry. J Chromatogr. B 1999, 733, 247–264. [Google Scholar] [CrossRef]
- Dams, R.; Murphy, C.M.; Lambert, W.E.; Huestis, M.A. Urine drug testing for opioids, cocaine, and metabolites by direct injection liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Siems, W.F.; Hill, H.H. Secondary electrospray ionization ion mobility spectrometry/mass spectrometry of illicit drugs. Anal. Chem. 2000, 72, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Asturias-Arribas, L.; Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Arcos-Martinez, M.J. CYP450 biosensors based on screen-printed carbon electrodes for the determination of cocaine. Anal. Chim. Acta 2011, 685, 15–20. [Google Scholar] [CrossRef]
- Sengel, T.Y.; Guler, E.; Gumus, Z.P.; Aldemir, E.; Coskunol, H.; Akbulut, H.; Colak, D.G.; Cianga, I.; Yamada, S.; Timur, S.; et al. An immunoelectrochemical platform for the biosensing of ‘Cocaine use’. Sens. Actuators B Chem. 2017, 246, 310–318. [Google Scholar] [CrossRef]
- Abdelshafi, N.A.; Bell, J.; Rurack, K.; Schneider, R.J. Microfluidic electrochemical immunosensor for the trace analysis of cocaine in water and body fluids. Drug Test. Anal. 2019, 11, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Eldefrawi, M.; Wright, J.; Darwin, D.; Huestis, M. A rapid reusable fiber optic biosensor for detecting cocaine metabolites in urine. J. Anal. Toxicol. 1999, 23, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Rauf, S.; Zhang, L.; Ali, A.; Liu, Y.; Li, J.H. Label-Free Nanopore Biosensor for Rapid and Highly Sensitive Cocaine Detection in Complex Biological Fluids. ACS Sens. 2017, 2, 227–234. [Google Scholar] [CrossRef]
- Wang, J.; Hou, J.; Zhang, H.C.; Tian, Y.; Jiang, L. Single Nanochannel-Aptamer-Based Biosensor for Ultrasensitive and Selective Cocaine Detection. ACS Appl. Mater. Interfaces 2018, 10, 2033–2039. [Google Scholar] [CrossRef]
- Aydindogan, E.; Balaban, S.; Evran, S.; Coskunol, H.; Timur, S. A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics. Biosensors 2019, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Eremenko, A.V.; Bauer, C.G.; Makower, A.; Kanne, B.; Baumgarten, H.; Scheller, F.W. The development of a non-competitive immunoenzymometric assay of cocaine. Anal. Chim. Acta 1998, 358, 5–13. [Google Scholar] [CrossRef]
- Halamek, J.; Makower, A.; Skladal, P.; Scheller, F.W. Highly sensitive detection of cocaine using a piezoelectric immunosensor. Biosens. Bioelectron. 2002, 17, 1045–1050. [Google Scholar] [CrossRef]
- Guler, E.; Bozokalfa, G.; Demir, B.; Gumus, Z.P.; Guler, B.; Aldemir, E.; Timur, S.; Coskunol, H. An aptamer folding-based sensory platform decorated with nanoparticles for simple cocaine testing. Drug Test. Anal. 2017, 9, 578–587. [Google Scholar] [CrossRef]
- Yanez-Sedeno, P.; Agui, L.; Villalonga, R.; Pingarron, J.M. Biosensors in forensic analysis. A review. Anal. Chim. Acta 2014, 823, 1–19. [Google Scholar] [CrossRef]
- Sun, B.; Qi, H.L.; Ma, F.; Gao, Q.A.; Zhang, C.X.; Miao, W.J. Double Covalent Coupling Method for the Fabrication of Highly Sensitive and Reusable Electrogenerated Chemiluminescence Sensors. Anal. Chem. 2010, 82, 5046–5052. [Google Scholar] [CrossRef]
- Bauer, C.G.; Eremenko, A.V.; Kuhn, A.; Kurzinger, K.; Makower, A.; Scheller, F.W. Automated amplified flow immunoassay for cocaine. Anal. Chem. 1998, 70, 4624–4630. [Google Scholar] [CrossRef]
- Tran, R.J.; Sly, K.L.; Conboy, J.C. Revealing the Kinetic Advantage of a Competitive Small-Molecule Immunoassay by Direct Detection. Anal. Chem. 2020, 92, 13163–13171. [Google Scholar] [CrossRef]
- Yu, H.; Kusterbeck, A.W.; Hale, M.J.; Ligler, F.S.; Whelan, J.P. Use of the USDT flow immunosensor for quantitation of benzoylecgonine in urine. Biosens. Bioelectron. 1996, 11, 725–734. [Google Scholar] [CrossRef]
- Swensen, J.S.; Xiao, Y.; Ferguson, B.S.; Lubin, A.A.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Soh, H.T. Continuous, Real-Time Monitoring of Cocaine in Undiluted Blood Serum via a Microfluidic, Electrochemical Aptamer-Based Sensor. J. Am. Chem. Soc. 2009, 131, 4262–4266. [Google Scholar] [CrossRef] [Green Version]
- Spiehler, V.; Fay, J.; Fogerson, R.; Schoendorfer, D.; Niedbala, R.S. Enzyme immunoassay validation for qualitative detection of cocaine in sweat. Clin. Chem. 1996, 42, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Crooks, C.R.; Brown, S. Roche DAT Immunoassay: Sensitivity and Specificity Testing for Amphetamines, Cocaine, and Opiates in Oral Fluid. J. Anal. Toxicol. 2010, 34, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Inoue, H.; Chikuma, T.; Itoh, J.; Makino, Y.; Hojo, H. A rapid enzyme immunoassay for cocaine and benzoylecgonine using glucose oxidase. J. Health Sci. 2001, 47, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Armenta, S.; de la Guardia, M. Analytical methods to determine cocaine contamination of banknotes from around the world. TrAC Trend Anal. Chem. 2008, 27, 344–351. [Google Scholar] [CrossRef]
- Dixon, S.J.; Brereton, R.G.; Carter, J.F.; Sleeman, R. Determination of cocaine contamination on banknotes using tandem mass spectrometry and pattern recognition. Anal. Chim. Acta 2006, 559, 54–63. [Google Scholar] [CrossRef]
- Song, D.M.; Zhang, S.D.; Kohlhof, K. Determination of a trace amount of cocaine on a bank note by gas chromatography positive-ion chemical-ionization mass spectrometry. J. Chromatogr. A 1996, 731, 355–360. [Google Scholar] [CrossRef]
- Troiano, G.; Mercurio, I.; Golfera, M.; Nante, N.; Melai, P.; Lancia, M.; Bacci, M. Cocaine contamination of banknotes: A review. Eur. J. Public Health 2017, 27, 1097–1101. [Google Scholar] [CrossRef]
- Abdelshafi, N.A.; Panne, U.; Schneider, R.J. Screening for cocaine on Euro banknotes by a highly sensitive enzyme immunoassay. Talanta 2017, 165, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.H.; Chen, L.F.; Luo, F.; Qiu, B.; Lin, Z.Y.; Chen, G.N. Determination of cocaine on banknotes through an aptamer-based electrochemiluminescence biosensor. Anal. Bioanal. Chem. 2011, 400, 289–294. [Google Scholar] [CrossRef]
- Bones, J.; Macka, M.; Paull, B. Evaluation of monolithic and sub 2 µm particle packed columns for the rapid screening for illicit drugs—application to the determination of drug contamination on Irish euro banknotes. Analyst 2007, 132, 208–217. [Google Scholar] [CrossRef]
- Luzardo, O.P.; Almeida, M.; Zumbado, M.; Boada, L.D. Occurrence of Contamination by Controlled Substances in Euro Banknotes from the Spanish Archipelago of the Canary Islands. J. Forensic Sci. 2011, 56, 1588–1593. [Google Scholar] [CrossRef]
- Paul, M.; Tscheuschner, G.; Herrmann, S.; Weller, M.G. Fast Detection of 2,4,6-Trinitrotoluene (TNT) at ppt Level by a Laser-Induced Immunofluorometric Biosensor. Biosensors 2020, 10, 89. [Google Scholar] [CrossRef]
- Weller, M.G.; Weil, L.; Niessner, R. Determination of Triazine Herbicides by ELISA—Optimization of Enzyme Tracer Synthesis. Fresenius J. Anal. Chem. 1992, 343, 51–52. [Google Scholar] [CrossRef]
- Wilke, M.; Röder, B.; Paul, M.; Weller, M.G. Sintered Glass Monoliths as Supports for Affinity Columns. Separations 2021, 8, 56. [Google Scholar] [CrossRef]
- Hoffmann, H.; Baldofski, S.; Hoffmann, K.; Flemig, S.; Silva, C.P.; Esteves, V.I.; Emmerling, F.; Panne, U.; Schneider, R.J. Structural considerations on the selectivity of an immunoassay for sulfamethoxazole. Talanta 2016, 158, 198–207. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsuda, R.; Maitani, T.; Imai, K.; Nishimura, W.; Ito, K.; Maeda, M. Precision, limit of detection and range of quantitation in competitive ELISA. Anal. Chem. 2004, 76, 1295–1301. [Google Scholar] [CrossRef]
- Paul, M.; Weller, M.G. Antibody Screening by Microarray Technology-Direct Identification of Selective High-Affinity Clones. Antibodies 2020, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Tscheuschner, G.; Schwaar, T.; Weller, M.G. Fast Confirmation of Antibody Identity by MALDI-TOF MS Fingerprints. Antibodies 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinorini, M.T.; Bernasconi, P.; Heeb, T.; Grata, E.; Capella, M.; Trachsel, A.; Santacroce, G.; Weinmann, W. Detection of cocaine on euro banknotes; Development of a practical approach for the interpretation of suspect cases. Forensic Sci. Int. 2020, 309, 110227. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, M.; Tannenberg, R.; Tscheuschner, G.; Ponader, M.; Weller, M.G. Cocaine Detection by a Laser-Induced Immunofluorometric Biosensor. Biosensors 2021, 11, 313. https://doi.org/10.3390/bios11090313
Paul M, Tannenberg R, Tscheuschner G, Ponader M, Weller MG. Cocaine Detection by a Laser-Induced Immunofluorometric Biosensor. Biosensors. 2021; 11(9):313. https://doi.org/10.3390/bios11090313
Chicago/Turabian StylePaul, Martin, Robert Tannenberg, Georg Tscheuschner, Marco Ponader, and Michael G. Weller. 2021. "Cocaine Detection by a Laser-Induced Immunofluorometric Biosensor" Biosensors 11, no. 9: 313. https://doi.org/10.3390/bios11090313





